
ijѧϰС�����λͬѧΪ�ⶨ��п��Ƥ�ĶƲ�ĺ�ȣ�����˸��Ե���Ʒ�����������п�Ʋ����������ͬѧ�ķ������������Ὣ��п��Ƥ�����п��Ӧ����ͨ�����������п��������Ȼ������п���ܶ����п�������������������Զ�п��Ƥ���������õ�п��ĺ�ȡ�
��1����ͬѧ�ķ����Ƿ���У�˵�����ɣ� ��
��ͬѧ�ķ�����ͨ���������ϣ�֪��Zn(OH)2�ȿ�������Ҳ����Ӧ��������������·�����
��2������5%������1 L (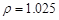 g/cm3 )����ȡ��36.5% (
g/cm3 )����ȡ��36.5% (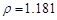 g/cm3 )������ mL
g/cm3 )������ mL
������һλС������
��3����ʹ�õĶ�п��Ƥ������Ϊ28.357g�����Ƶ����պ���������Ϊ40.000g����п��Ƥ�ij�5.00cm����5.00cm��п���ܶ�Ϊ7.14g/cm3����п��ĺ��Ϊ cm����ͬѧ�ķ�����ͨ����ͼ��ʾװ�ã�������п��Ƥ��ϡH2SO4��Ӧ�������������������п��ĺ�ȡ����Ƶö�п��Ƥ����Ϊ18.200g��
��4��ʵ�����ó�������Ϊ ��
��5��������Ũ���ᣬ����п�ĺ�Ȼ� ���ƫ����ƫС��������Ӱ�족����
��6��ʵ�����ͬѧ�ͱ�ͬѧ�Ľ�����бȽϣ��������Ƕ�ͬ�ֶ�п��Ƥ�IJ����������ܴ�����Ϊ˭�ķ������ӿɿ��أ� �����ǣ� ��
��1�������� (1��) FeҲ������ᷴӦ (1��)
��2��118.9 (2��) ��3��0.001 (2��)
��4��������ƽ (2��) ��5��ƫС (2��)
��6���� (1��) ���ķ�������������ˮ����������ɸ��š�(1��)
�������������
��2���⣺����36.5% (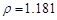 g/cm3 )���������ΪX
g/cm3 )���������ΪX
�������ʲ��䣺1.025*1000*5%=1.181*X*36.5% X=118.9
��3���⣺����������Ϊy
2Fe------- Fe2O3
112 160
y 40.0 y="28"
п��ĺ��:(m��-m��)/pп/5*5="(28.357-28)/" 7.14g*5*5=0.002��cm����Ϊ���������棬���涼��0.001 cm.
��5��ƫС����Ϊ����ӷ�����ʧһ�����ᣬ�������������п��С��
��6�����ķ����Ǹ���������������п������������ˮ��������ɵ����������ܴ�
���㣺�����Ի�ѧʵ��Ϊ����������ʵ����ơ�ʵ������������ͻ�ѧ�����֪ʶ��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
K3[Fe��C2O4��3]��3H2O[���������������ؾ���]������ˮ���������Ҵ�������Ϊ�л���Ӧ�Ĵ�����ʵ���ҿ�����мΪԭ���Ʊ�����ط�Ӧ�������¡���ش��������⣺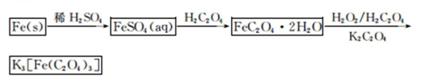
��1����м�г�����Ԫ�أ�������Ʊ�FeSO4ʱ������ж���H2S���壬�������������������Һ���ա���������װ����ȷ���� ������ţ���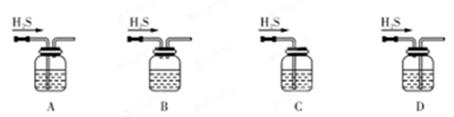
��2���ڵõ���FeSO4��Һ�������������H2 SO4�ữ��Ŀ���� ���õ�K3[Fe(C2O4)3]��Һ�����Ҵ���Ŀ���� ��
��3�������������ᾧˮ��ͨ�������������ⶨ����Ҫ�����У��ٳ����������ں������ѽᾧˮ������ȴ���ܳ��������ظ��ڡ��������أ����㡣
����ݵ�Ŀ���� ��
��4��C2O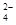 �ɱ�����KMnO4��Һ����ΪCO2���壬��ʵ�������K3[Fe��C2O3��3]��3H2O�����ⶨ����KMnO4����Һ�ζ���
�ɱ�����KMnO4��Һ����ΪCO2���壬��ʵ�������K3[Fe��C2O3��3]��3H2O�����ⶨ����KMnO4����Һ�ζ���
��д���ζ������з�����Ӧ�����ӷ���ʽ ��
�����еζ�������ʹ�ζ����ƫ�ߵ��� ������ţ���
| A���ζ���������ˮϴ�Ӻ�����װ���Һ |
| B����ƿ��װ����Һǰδ�ô���Һ��ϴ |
| C���ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ���������ʧ |
| D����ȡ��Һ���ʱ���ζ�ǰ���Ӷ������ζ����Ӷ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ���Һϳ����������IJ������£�
��Բ����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װͨ����ȴˮ��������(ʹ��Ӧ��������������ΪҺ��������ƿ��)�����Ȼ���һ��ʱ�������װ�ý������õ������Ҵ��������ˮ�����������ֲ�Ʒ��
��ش��������⣺
��1������ƿ�г��˼������ᡢŨ������Ҵ��⣬��Ӧ�������Ƭ��Ŀ����__________________��
��2����Ӧ�м���������Ҵ���Ŀ����________________________________________________��
��3�����������ʵ�鲽���Ϊ��������ƿ���ȼ����Ҵ���Ũ���ᣬȻ��ͨ����Һ©���ߵμ����ᣬ��������������������������IJ��ʣ���ԭ����_____________________��
��4���������ֲ�Ʒ����������������Ҵ��Ļ�����ͼ�Ƿ��������������ͼ��
���Լ�a�ǣ�________�����뷽�����ǣ�________________________�����뷽�����ǣ�____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ա���ۣ���Ҫ�ɷ�ΪBaCO3������Ca2+��Fe2+��Fe3+��Mg2+�ȣ��Ʊ�BaCl2��2H2O���������£�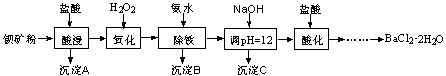
��1������������Ҫ��Ӧ�����ӷ���ʽΪ ��
��2������C����Ҫ�ɷ���Ca(OH)2�� ��
��ͼ��֪��Ϊ�˸��õ�ʹCa2+��������Ӧ��ȡ�Ĵ�ʩΪ ��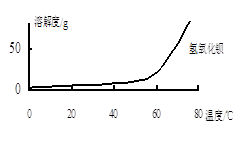
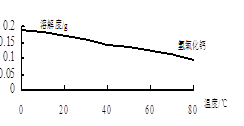
��3����BaSO4�������ⶨ��Ʒ���ȵIJ���Ϊ��
����1��ȷ��ȡ0.4��0.6 g BaCl2��2H2O����������100 ml ˮ��3 ml 2 mol��L-1��HCl��Һ�����ܽ⡣
����2���߽��裬����μ���0.1 mol��L-1 H2SO4��Һ��
����3����BaSO4������ ��ȷ������ȫ������
����4�����ˣ���0.01 mol��L-1��ϡH2SO4ϴ�ӳ���3~4�Σ�ֱ��ϴ��Һ�в���Cl��Ϊֹ��
����5�����۵��ij�����ֽ������ �У�����ɡ�̿�����һ�����800�����������ء���������BaCl2��2H2O��Ba2+�ĺ�����
�ٲ���3��ȱ�IJ���Ϊ ��
��������1��������Ʒ���٣����ڲ���4ϴ��ʱ������ɵ�Ӱ��Ϊ ��
�۲���5���ô�����������Ϊ ����ֽ�һ�ʱ����Ҫ���㣬����BaSO4�ױ�������̿��ԭ����BaS���÷�Ӧ�Ļ�ѧ����ʽΪ ��
����ͬѧ��Ϊ��K2CrO4����H2SO4��������Ч�����ã���˵��ԭ�� ��
[��֪��Ksp(BaSO4)=1.1��10-10 Ksp(BaCrO4)=1.2��10-10]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�̷����壨FeSO4��7H2O��M=278g/mol��������ȱ����ƶѪҩƷ����Ҫ�ɷ֡�ʵ�����������᳧����������Ҫ�ɷ�ΪFe2O3������FeS��SiO2�����Ʊ��̷��Ĺ������£�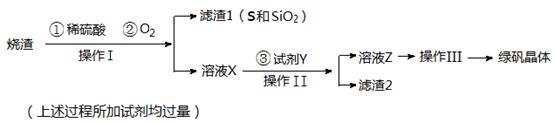 �Իش�
�Իش�
��1��������Ϊ ����д�������ƣ���
��2�� �Լ�Y����ҺX��Ӧ�����ӷ���ʽΪ ��
��3�����������̷������к���Fe2+��ʵ������� ��
��4���������˳������Ϊ�� ����ȴ�ᾧ������ �� �����
��5��ijͬѧ������KMnO4��Һ�ⶨ�̷���Ʒ��Fe2+������
a.��ȡ11.5g�̷���Ʒ���ܽ⣬���Ƴ�1000mL��Һ��
b.��ȡ25.00mL������Һ����ƿ�У�
c.�������ữ��0.01000mol/L KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL��
�ٲ���a������Һʱ��Ҫ�IJ�������������������Ͳ���ձ�����ͷ�ι��⣬���� ��
�ڸ�ͬѧ��Ƶ����еζ���ʽ����������� (�гֲ�����ȥ)(����ĸ���)��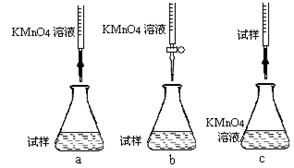
�۵ζ�ʱ������Ӧ�����ӷ���ʽΪ�� ��
���жϴ˵ζ�ʵ��ﵽ�յ�ķ����� �����ڵζ��յ��ȡ�ζ��̶ܿ�ʱ������KMnO4��ҺҺ�棬������������ȷ����ʹ�ⶨ��� ���ƫ�ߡ���ƫ�͡�����Ӱ�족����
�ݼ���������Ʒ��FeSO4��7H2O����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��̼���ƣ�2Na2CO3��3H2O2����һ�ּ�ϴ�ӡ�Ư�ס�ɱ����һ�����ϵƯ�����þ������Na2CO3��H2O2��˫�����ʡ�����ͼ-2װ���Ʊ���̼���ƣ�����ˮԡ�г�ַ�Ӧ��ͼ-1���̿ɻ�ù�̼���Ʋ�Ʒ��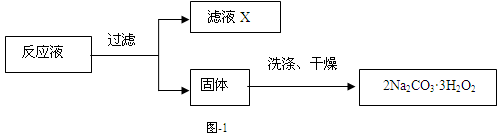
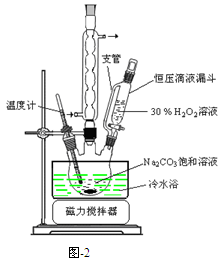
���������գ�
��1����ѹ��Һ©����֧�ܵ������� ��
��2���Ʊ���̼���ƵĹؼ��� ��
��3������ʹ��̼����ʧЧ��������_______��ѡ���ţ���
a��Na2S b��CH3COOH c��NaHCO3
��̼���Ʋ�Ʒ��������������̼���ƣ������������ⶨ��̼���Ƶ�����������
��4�����ʵ�鲽�裺
�ܽ� �� ������Ӧ �� ________ �� ________ �� ________ �� ________ ��
��5��д��������Ӧ�����ӷ���ʽ________________________________________��
��6����Ҫֱ�Ӳⶨ���������У�________________________________������ĸ��ʾ��ע���京�壩����Ʒ�й�̼�������������ı���ʽΪ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ҵ�Ƶõĵ�����(AlN)��Ʒ�г���������Al4C3��Al2O3��C�����ʡ�ijͬѧ���������ʵ��ֱ�ⶨ������(AlN)��Ʒ��AlN��Al4C3����������(����NH3��ǿ������Һ�е��ܽ�)��
��1��ʵ��ԭ��
��Al4C3�����ᷴӦ������CH4��
��AlN����ǿ�������Σ�����ǿ�����ɰ�������д��AlN��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
��2��ʵ��װ��(��ͼ��ʾ)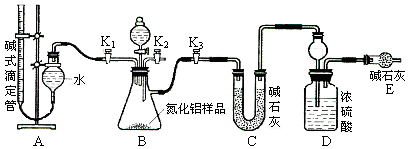
��3��ʵ�����
������ʵ��װ�ã�����װ�õ������ԡ��Ƶ�Dװ�õ�����Ϊyg���ζ��ܵĶ���ΪamL��
�ڳ�ȡxg AlN��Ʒ������ƿ�У����ý������رջ��� ������ ��ͨ����Һ©������ϡ���ᣬ����ƿ�����ʳ�ַ�Ӧ��
�۴���Ӧ������ȫ�رջ��� ������ ��ͨ����Һ©��������� (�ѧʽ)������ƿ�����ʳ�ַ�Ӧ��
�� (����ò�Ӧ���еIJ���)��
�ݼ�¼�ζ��ܵĶ���ΪbmL���Ƶ�Dװ�õ�����Ϊzg��
��4�����ݷ���
��AlN����������Ϊ ��
������ȡ�ζ�������������ʱ��Һ������ҵͣ��������������� (�ƫ����ƫС������Ӱ�족)��
��Al4C3����������Ϊ ��(��ʵ�������µ�����Ħ�����ΪVm)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����6����ɫ��Һ���Ҵ������ӡ�Na2CO3��Һ��AgNO3��Һ��KOH��Һ�������ᣬ���ֵ��Լ���
| A�����Ƽ���Cu(OH)2����Һ | B��FeCl3��Һ |
| C��BaCl2��Һ | D������KMnO4��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���г��ӷ�����ȷ����
| A����������ȥ�����л��е�������ϩ |
| B���ý����Ƴ�ȥ�Ҵ��л��е�����ˮ |
| C�������Ը��������Һ��ȥ�����л��е�������ϩ |
| D���ñ���̼��������Һ��ȥ������̼�л��е������Ȼ��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com