
ij��ҵ��ˮ�к���CN-��Cr2O �����ӣ��辭��ˮ������������ŷţ���ˮ�����������������̽��д�����
�����ӣ��辭��ˮ������������ŷţ���ˮ�����������������̽��д�����
�ش��������⣺
��1��������У�CN-��ClO-����ΪCNO-�����ӷ���ʽΪ________________��
��2������۵ķ�ӦΪS2O32����Cr2O72����H�� SO42����Cr3����H2O��δ��ƽ������ÿ����0.4mol Cr2O72��ת��__________mol e-��
SO42����Cr3����H2O��δ��ƽ������ÿ����0.4mol Cr2O72��ת��__________mol e-��
��3����Cr3+��ˮ���Լ�����ʯ�ҽ�һ��������Ŀ����____________________��
��4����25���£���amol/L��NaCN��Һ��0.01mol/L������������ϣ���Ӧ������ҺpH��7����a________0.01�����������������=�������ú�a�Ĵ���ʽ��ʾHCN�ĵ��볣��Ka��_________________��
��5��ȡ��ҵ��ˮˮ�����Թ��У�����NaOH��Һ�۲쵽����ɫ�������ɣ������������ٲ�����ɫ����Ϊֹ��������Һ�м�������Na2S��Һ����ɫ����ת���ɺ�ɫ�������ù����з�Ӧ�����ӷ�����________��
��1��CN����ClO����CNO����Cl����2�֣� ��2�� 2.4��2�֣�
��3�����ڷ�ˮpH��ʹ��ת����Cr(OH)3������ȥ��2�֣����������𰸾����֣�
��4������2�֣� (100a��1)��10��7 mol��L��1��2�֣���λ��дҲ���֣�
��5��Cu2����2OH����Cu(OH)2����2�֣���
Cu(OH)2(s)��S2��(aq)��CuS(s)��2OH��(aq)��2�֣���ע��״̬Ҳ���֣�
���������������1��������У�CN����ClO������ΪCNO������Ϊ���ڼ��Ի����У�����ClO��ֻ�ܱ���ԭΪCl������˷�Ӧ�����ӷ���ʽΪCN����ClO����CNO����Cl����
��2�����ݷ���ʽ��֪���ڷ�Ӧ��CrԪ�صĻ��ϼ۴ӣ�6�۽��͵���3�ۣ��õ�3�����ӣ�����0.4 mol Cr2O72-ת��ΪCr3��ʱת�Ƶ��ӵ����ʵ�����0.4mol����6��3����2��2.4mol��
��3����ʯ����ǿ�����ˮ�Լ��ԣ����Ժ�Cr3+��ˮ�м�����ʯ�ҽ�һ��������Ŀ���ǵ��ڷ�ˮpH��ʹ��ת����Cr(OH)3������ȥ��
��4��NaCN�����ᷴӦ����HCN���Ȼ��ƣ���Ӧ������ҺpH��7����˵��NaCN���������a��mol/L�����ݵ���غ�c(Na��)��c(H��)��c(OH��)��c(Cl��)��c(CN��)�������غ�c(Na��)��c(CN��)��c(HCN)��֪��c(HCN)��c(Cl��)��0.005mol/L��c(CN��)��0.5amol/L��0.005mol/L�����Ը��¶���HCN�ĵ��볣��Ka��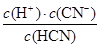 ��
�� ��(100a��1)��10��7 mol��L��1��
��(100a��1)��10��7 mol��L��1��
��5����ɫ����ΪCu(OH)2��CuS(s)��Cu(OH)2(s)�����ܣ������˳�����ת�������Է�Ӧ�Ļ�ѧ����ʽΪCu2����2OH����Cu(OH)2����Cu(OH)2(s)��S2��(aq)��CuS(s)��2OH��(aq)��
���㣺����������ԭ��Ӧ�����ӷ���ʽ����д��ˮ��Һ�е�����ƽ�⡢����Ũ�ȡ����볣���������ܽ�ƽ��ȵ������Һ�����֪ʶ



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)25 ��ʱ��0.1 mol��L��1NaOH��Һ��pHΪ________��
(2)25 ��ʱ��0.1 mol��L��1NH4Cl��Һ��pH_____7(�����������������)����ԭ����_________(�����ӷ���ʽ��ʾ)��
(3)������������Һ�������Ϻ�����Ũ�ȴ�С������ȷ����________(�����)��
A��[Na��]��[Cl��]��[OH��]��[H��]
B��[Na��]��[Cl��]��[H��]��[OH��]
C��[Na��]��[Cl��]��[OH��]��[H��]
D��[Cl��]��[Na��]��[OH��]��[H��]
(4) ���������ܵ��������ڵ���ʵ���________(�����)��
A.�� B.���� C.�Ȼ��ƾ��� D.������������
(5) 25��ʱ��0.1 mol��L��1NaOH��Һ��ˮ�����������������Ũ��ΪC1��0.1 mol��L��1NH4Cl��Һ��ˮ��������������ӵ�Ũ��ΪC2����C1 ______C2(�����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪MOHΪһԪ���25��ʱ�����볣��Kb= 1��10�� 6mol��L��1��
��1��25��ʱ����0.2 mol��L��1 HCl��Һ��0.2 mol��L��1 MOH��Һ��������(���Ի�Ϻ���Һ����ı仯)����û����Һ��pH��6����ʱ�����Һ����ˮ�������c(H��)=Amol��L��1����0.2 mol/L HCl��Һ����ˮ�������c(H��)=Bmol��L��1����
�ٱȽ�A B��(�>������<������)
�ڸ��ݵ���غ㣬��������Һ��c(Cl��)��c(M��)��______ mol��L��1�� (��ȷ���㣬���������)
��2��25��ʱ��0.01 mol��L��1MOH��Һ�� pH=10������������ pH = 4��������Һ��ϣ�������Һ��pH 7���>������<����=�����������ʱ��Һ��M Cl��ˮ��ƽ�ⳣ��Kh= mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����£���ijһԪ��BOH��HCl��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
| ʵ���� | HCl�����ʵ���Ũ�� ��mol��L-1�� | BOH�����ʵ���Ũ�� ��mol��L-1�� | �����Һ��pH |
| �� | 0.1 | 0.1 | pH=5 |
| �� | c | 0.2 | pH=7 |
| �� | 0.1 | 0.2 | pH>7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ס�����ͬѧ����ʵ��ȷ��ij��HA��������ʡ����ǵķ����ֱ��ǣ�
�ף��ٳ�ȡһ��������HA����0.1 mol/L����Һ100 mL��
����pH��ֽ�������Һ��pH������֤��HA��������ʡ�
�ң�������֪���ʵ���Ũ�ȵ�HA��Һ�����ᣬ�ֱ�����pH��1����������Һ��100 mL��
�ڷֱ�ȡ��������Һ��10 mL����ˮϡ��Ϊ100 mL��
�۸�ȡ��ͬ���������ϡ��Һװ�������Թܣ�ͬʱ���봿����ͬ��п�����۲�������֤��HA��������ʡ�
��1�������������ĵڢٲ��У���Ҫ�õ��Ķ��������� ��
��2�������У�˵��HA�������ʵ������Dz����Һ��pH 1��ѡ�����������������������Ҫ˵��pH��ֽ��ʹ�÷����� ��
��3���ҷ����У�˵��HA��������ʵ�������
��
��4�����������һ���������Ƚ������еķ�����ҩƷ����ȡ��������������Ҫ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���Ի�ͭ��(��Ҫ�ɷ���CuFeS2�����ʲ�����ˮ����)Ϊԭ�ϣ��Ʊ���ɫ����G���仯ѧʽΪ[Cu(NH3)4]SO4��H2O���漰�������£�
��֪25��ʱ�����ֽ�������������ܶȻ���������ȫ������pH��Χ���±���
| | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| Ksp | 8.0��10-16 | 2.2��10-22 | 4.0��10-38 |
| ��ȫ����pH | ��9.6 | ��6.4 | ��3.2 |
 [Cu(NH3)4]2++2OH-+4H2O��д���÷�Ӧ��ƽ�ⳣ������ʽ�� ��
[Cu(NH3)4]2++2OH-+4H2O��д���÷�Ӧ��ƽ�ⳣ������ʽ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij���Ṥ�������Է�ˮ���飨As��Ԫ�غ������ߣ�Ϊ��������ŷţ����û�ѧ���������������ˮ�������������£�
����������±�����ش��������⣺
��1�������ε�Ksp
| ������ | Ksp |
| Ca3(AsO4)2 | 6.8��10��19 |
| CaSO4 | 9.1��10��6 |
| FeAsO4 | 5.7��10��21 |
| ��Ⱦ�� | H2SO4 | As |
| ��ˮŨ�� | 29.4g/L | 1.6g��L��1 |
| �ŷű� | pH 6��9 | 0.5mg��L��1 |
 HAsO42��+OH�����ò�ˮ���ƽ�ⳣ����25�棩Ϊ�� ��������λ��Ч���֣���
HAsO42��+OH�����ò�ˮ���ƽ�ⳣ����25�棩Ϊ�� ��������λ��Ч���֣����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
���ʼ��仯�����ڹ�ũҵ������������Ҫ��Ӧ�á�
��1����֪25��ʱ��
SO2��g����2CO��g����2CO2��g����1/xSx��s�� ��H��akJ/mol
2COS��g����SO2��g����2CO2��g����3/xSx��s�� ��H��bkJ/mol��
��COS��g������CO��g����Sx��s����Ӧ���Ȼ�ѧ����ʽ�� ��
��2���ۻ�(As4S4)�ʹƻ�(As2S3)����ȡ�����Ҫ����ԭ�ϡ���֪As2S3��HNO3�����·�Ӧ��
As2S3+10H++ 10NO3?=2H3AsO4+3S+10NO2��+ 2H2O��������H3AsO4�����ʵ���
Ϊ0.6 mol��Ӧ��ת�Ƶ��ӵ���ĿΪ ��
��3��������ʵ���Ũ��Na2S��NaOH�����Һ�еμ�ϡ����������������H2S��HS?��S2?�ķֲ�������ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ�������μ���������Ĺ�ϵ����ͼ��ʾ�����Եμӹ���H2S������ݳ�����
��B��ʾ ��
�ڵμӹ����У���Һ����Ũ�ȴ�С��ϵ��ȷ���� (����ĸ)�� a��c(Na+)= c(H2S)+c(HS?)+2c(S2?)
a��c(Na+)= c(H2S)+c(HS?)+2c(S2?)
b��2c(Na+)=c(H2S)+c(HS?)+c(S2?)
c��c(Na+)=3[c(H2S)+c(HS?)+c(S2?)]
��NaHS��Һ�ʼ��ԣ����μ�������M��ʱ����Һ�и�����Ũ���ɴ�С��˳��Ϊ  ��4����ҵ������·ѭ����������������Ĺ�����������ͼ��ʾ��
��4����ҵ������·ѭ����������������Ĺ�����������ͼ��ʾ��
�� д����Ӧ���з�����Ӧ�Ļ�ѧ����ʽ�� ��
�� ������װ������ͼ��ʾ��д�������ĵ缫��Ӧʽ ����װ���з������ܷ�Ӧ�Ļ�ѧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС��Ϊ��̽������ĵ������������������ʵ�顣
ʵ��һ�����Ʋ��궨������Һ��Ũ��
ȡ����������250 mL 0.2 mol��L��1�Ĵ�����Һ����0.2 mol��L��1�Ĵ�����Һϡ�ͳ�����Ũ�ȵ���Һ������NaOH����Һ�����������Һ��Ũ�Ƚ��б궨���ش��������⣺
(1)����250 mL 0.2 mol��L��1������Һʱ��Ҫ�õ��IJ�����������Ͳ���ձ�����������__________________��______________��
(2)Ϊ�궨ij������Һ��ȷŨ�ȣ���0.200 0 mol��L��1��NaOH��Һ��20.00 mL������Һ���еζ������εζ�����NaOH��Һ��������£�
| ʵ����� | 1 | 2 | 3 | 4 |
| ����NaOH��Һ�����(mL) | 20.05 | 20.00 | 18.80 | 19.95 |
| ������ҺŨ��(mol��L��1) | 0.001 0 | 0.010 0 | 0.020 0 | 0.100 0 | 0.200 0 |
| pH | 3.88 | 3.38 | 3.23 | 2.88 | 2.73 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com