
�����£���ijһԪ��BOH��HCl��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
| ʵ���� | HCl�����ʵ���Ũ�� ��mol��L-1�� | BOH�����ʵ���Ũ�� ��mol��L-1�� | �����Һ��pH |
| �� | 0.1 | 0.1 | pH=5 |
| �� | c | 0.2 | pH=7 |
| �� | 0.1 | 0.2 | pH>7 |
��12�֣���ÿ��2�֣�
��1�� ��� 1��10��5
��2��<�� =
��3�� >�� =
���������������1�������ʵ�����һԪ�ᡢ����ʱ��Һ�����ԣ�������������Һ�������Ӿ�����������ˮ��õ��ӵģ�������Һ��ˮ�������c��OH����=c��H����=1��10-5mol��L��1���ʴ�Ϊ�����1��10-5��
��2��BOHΪ����������Ũ�Ȼ����Һ��pHС��7����Ϊ��֤pH=7��Ӧʹ��Ũ��С��0.2mol��L��1����Һ�����ԣ���Һ��c��OH����=c��H��������Һ�ʵ����ԣ����ڵ���غ㣬����c��B����=c��Cl�������ʴ�Ϊ������=��
��3���ɢ���ʵ������֪����Ϻ�ΪBOH��BCl�Ļ��Һ��pH��7����ĵ�������ε�ˮ�⣬��Һ�д��ڵ���غ�c��H����+c��B�� ��=c��OH����+c��Cl�� �������������غ��c��B����+c��BOH��=2c��Cl�� ������������ʽ������c��B����-2c ��OH����=c��BOH��-2c��H�������ʴ�Ϊ������=��
���㣺�����ʱ�Ķ����жϼ��й�pH�ļ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��������ʱH2O H++OH-��KW=10-14�� CH3COOH
H++OH-��KW=10-14�� CH3COOH  H++ CH3COO����Ka=1.8��10-5
H++ CH3COO����Ka=1.8��10-5
��1��ȡ����������Һ���������������ƹ��壬��ʱ��Һ��C��H+����C��CH3COOH���ı�ֵ�� �� �������С�����䡱��
��2��������ˮ������ӷ���ʽΪ�������� �������� ���������¶�ʱ��C(OH��)�������������������С�������䡱����
��3��0��5mol��L-1��������ҺpHΪm����ˮ��ij̶ȣ���ˮ��Ĵ�������ԭ�д����Ƶı�ֵ��Ϊa��1mol��L-1��������ҺpHΪn��ˮ��ij̶�Ϊb����m��n�Ĺ�ϵΪ�������� ��a��b�Ĺ�ϵΪ����������������ڡ���С�ڡ������ڡ�����
��4�����������Ũ�ȵĴ��������������Һ��Ϻ�������Һ������Ũ���ɴ�С��˳�������������������� ������ ��
��5�������������������Һ��Ϻ�pH<7����c��Na+��_______________ c��CH3COO����������ڡ�����С�ڡ����ڡ�����
��6������ʱ������pH��3��HA��ҺV1mL��pH��11��NaOH��ҺV2 mL��ϣ�������˵������ȷ����____________��
| A������Ӧ����Һ�����ԣ���c��H+��+c��OH������2��10��7mol��L��1 |
| B����V1=V2����Ӧ����ҺpHһ������7 |
| C������Ӧ����Һ�����ԣ���V1һ������V2 |
| D������Ӧ����Һ�ʼ��ԣ���V1һ��С��V2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ش������й�Na2S��Һ�����⡣
��1��Na2S��Һʢװ�ڴ��������Լ�ƿ�У���ϸ���г�������ζ���������ӷ���ʽ���ͣ�
�� ��
��2����Na2S��Һ����AlCl3��Һ�У��а�ɫ�����ͳ�������ζ���������ɣ����������ӷ�ӦΪ��
��
��3����Na2S��Һ����AgCl����Һ�У����ɵĺ�ɫ������ ��д��ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������±ʯ(K2SO4��MgSO4��2CaSO4��2H2O)���ڡ�������ˮ�д��������ܽ�ƽ�⣺
K2SO4��MgSO4��2CaSO4��2H2O(s) 2Ca2����2K����Mg2����4SO42-��2H2O��Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�
2Ca2����2K����Mg2����4SO42-��2H2O��Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�
��1��������Ҫ�ɷ���________��CaSO4�Լ�δ����±ʯ��
��2���û�ѧƽ���ƶ�ԭ������Ca(OH)2��Һ���ܽ���±ʯ����K����ԭ��
��
��3�������ӡ������У��ȼ��� ��Һ��������Ȳ������ˣ��ټ��� ��Һ����ҺpH�����ԡ�
��4����ͬ�¶��£�K���Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ����ͼ����ͼ�ɵã������¶����ߣ�
�� ��
�� ��
���ܽ�����K����ƽ��Ũ������
��5�������Կ�����̼����Ϊ�ܽ��������ܽ������лᷢ����CaSO4(s)��CO32- CaCO3(s)��SO42-����֪298 Kʱ��Ksp(CaCO3)��2.80��10��9��Ksp(CaSO4)��4.90��10��5��������¶��¸÷�Ӧ��ƽ�ⳣ����K�� ��
CaCO3(s)��SO42-����֪298 Kʱ��Ksp(CaCO3)��2.80��10��9��Ksp(CaSO4)��4.90��10��5��������¶��¸÷�Ӧ��ƽ�ⳣ����K�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����£����ȡ0��1 mol/L HA��Һ��0��1 mol/L NaOH��Һ�������ϣ����Ի�Ϻ�
��Һ����ı仯������û����Һ��pH=8,�Իش��������⣺
(1)�����Һ��pH=8��ԭ�� �������ӷ���ʽ��ʾ����
(2)�����Һ����ˮ�������c(H+) ���>����<����=����0��1 mol/L NaOH
��Һ����ˮ�������c(H+)��
(3)������Һ��������ʽ�ľ�ȷ����������������֣���
c(Na+����c(A-���� mol/L,
c(OH-����c(HA) �� mol/L��
(4)��֪NH4A��ҺΪ���ԣ���֪HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶϣ�NH4)2CO3
��Һ��pH ������ڡ���С�ڡ����ڡ�)7����ͬ�¶��µ�Ũ�ȵ���
������Һ��
A��NH4HCO3 B��NH4A C��(NH4)2SO4 D��NH4Cl
��pH�ɴ�С��˳�������� ������ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ҹ�ij���͵��ͭ������ҵ����ұ��������ͭ��������ʴﵽ97����87������ͼ��ʾ��ұ���ӹ������̣�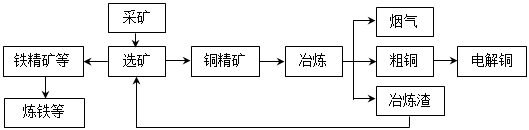
ұ���е���Ҫ��Ӧ��Cu2S + O2 =" 2Cu" + SO2
��1�������е���Ҫ������________________���������Դ�����ʺͼ��ſ��ǣ����ۺ����÷�ʽ����___________��
��2����ⷨ��ͭʱ��������____________�����ͭ�塱��ͭ�塱������ͭ�к��еĽ����Ե��ʵ���ʽ�ڵ���_______________������������������IJ۵ף������ĵ缫��Ӧʽ��_________________________________________��
��3���ھ���ͭ�Ĺ����У��������Һ��c(Fe2+)��c(Zn2+)���������Ӱ���һ����⡣
�������ʵ��ܶȻ�������KSP����
| ���� | Fe(OH)2 | Fe(OH)3 | Zn(OH)2 | Cu(OH)2 |
| KSP | 8.0��10��16 | 4.0��10��38 | 3.0��10��17 | 2.2��10��20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ҵ��ˮ�к���CN-��Cr2O �����ӣ��辭��ˮ������������ŷţ���ˮ�����������������̽��д�����
�����ӣ��辭��ˮ������������ŷţ���ˮ�����������������̽��д�����
�ش��������⣺
��1��������У�CN-��ClO-����ΪCNO-�����ӷ���ʽΪ________________��
��2������۵ķ�ӦΪS2O32����Cr2O72����H�� SO42����Cr3����H2O��δ��ƽ������ÿ����0.4mol Cr2O72��ת��__________mol e-��
SO42����Cr3����H2O��δ��ƽ������ÿ����0.4mol Cr2O72��ת��__________mol e-��
��3����Cr3+��ˮ���Լ�����ʯ�ҽ�һ��������Ŀ����____________________��
��4����25���£���amol/L��NaCN��Һ��0.01mol/L������������ϣ���Ӧ������ҺpH��7����a________0.01�����������������=�������ú�a�Ĵ���ʽ��ʾHCN�ĵ��볣��Ka��_________________��
��5��ȡ��ҵ��ˮˮ�����Թ��У�����NaOH��Һ�۲쵽����ɫ�������ɣ������������ٲ�����ɫ����Ϊֹ��������Һ�м�������Na2S��Һ����ɫ����ת���ɺ�ɫ�������ù����з�Ӧ�����ӷ�����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��12�֣�D��A��B��CΪ����ԭ��������������Ķ�����Ԫ�أ�A��B��Cͬ���ڣ�A��ԭ�Ӱ뾶��ͬ���������ģ�B��Dͬ���塣��֪DԪ�ص�һ�ֵ������ճ���������ˮ�����õ���������CԪ�صĵ��ʿ��Դ�A��B��Ԫ����ɵĻ������ˮ��Һ���û���BԪ�صĵ��ʡ�
��1��CԪ�������ڱ��е�λ�� ���� �塣
��2��A��BԪ���γɵij���������ˮ��Һ�� �ԣ�ԭ���ǣ������ӷ���ʽ��ʾ�� ��ʯī���缫���û������ˮ��Һ����������ӦʽΪ ��
��3��A��DԪ�ؿ����γɻ�����A2D2��д��A2D2��CO2��Ӧ�Ļ�ѧ����ʽ ����Ԫ�ط��ű�ʾ�����÷�Ӧ�л�ԭ���� ��
��4��BԪ�صĵ����ڲ�ͬ�������¿�����O2����һϵ�з�Ӧ���� B(s)+O2(g)=BO2(g)����H=��296.8kJ/mol��2BO2(g)+O2(g)  2BO3(g)����H=��196.6kJ/mol
2BO3(g)����H=��196.6kJ/mol
��1 mol BO3(g)����ȫ�ֽ��B��s������Ӧ�����е���ЧӦΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��14�֣�
�������ڹ�ҵ��ҽҩ���������Ź㷺����;����ͼ��ij��ȤС��ģ����Ʊ��������Ʒ�����Ƶ��������£�
��1����Ϣ�ʹ�ñ�ˮ��Ŀ���� ��
��2������II��III�������� , ��
��3����Ϣ��з�����Ӧ�����ӷ���ʽΪ ��
��4����ҵ����������ʹ���ʯ�Ƶ��廯���к�������Al3+��Fe3+���ʣ������������Լ� ���ѧʽ���������Һ��PHԼΪ8.0���ɳ�ȥ���ʣ�������Һ��PHԼΪ8.0��Ŀ����_______________________________________________________��
��5��t��ʱ����HBrͨ��AgNO3��Һ�����ɵ�AgBr��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪t��ʱAgCl��Ksp=4��l0-10������˵������ȷ���� �� ��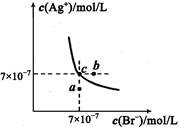
| A������Cl-��Br- �Ļ��Һ�еμ���������Һ��һ���Ȳ���AgBr�ij��� |
| B����AgBr������Һ�м���NaBr���壬��ʹ��Һ��c�㵽b�� |
| C��ͼ��a���Ӧ����AgBr�IJ�������Һ |
D����t��ʱ��AgCl(s)+Br-(aq) AgBr(s)+Cl-(aq)ƽ�ⳣ������816 AgBr(s)+Cl-(aq)ƽ�ⳣ������816 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com