
��14�֣�
�������ڹ�ҵ��ҽҩ���������Ź㷺����;����ͼ��ij��ȤС��ģ����Ʊ��������Ʒ�����Ƶ��������£�
��1����Ϣ�ʹ�ñ�ˮ��Ŀ���� ��
��2������II��III�������� , ��
��3����Ϣ��з�����Ӧ�����ӷ���ʽΪ ��
��4����ҵ����������ʹ���ʯ�Ƶ��廯���к�������Al3+��Fe3+���ʣ������������Լ� ���ѧʽ���������Һ��PHԼΪ8.0���ɳ�ȥ���ʣ�������Һ��PHԼΪ8.0��Ŀ����_______________________________________________________��
��5��t��ʱ����HBrͨ��AgNO3��Һ�����ɵ�AgBr��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪t��ʱAgCl��Ksp=4��l0-10������˵������ȷ���� �� ��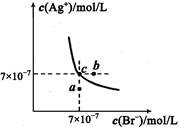
| A������Cl-��Br- �Ļ��Һ�еμ���������Һ��һ���Ȳ���AgBr�ij��� |
| B����AgBr������Һ�м���NaBr���壬��ʹ��Һ��c�㵽b�� |
| C��ͼ��a���Ӧ����AgBr�IJ�������Һ |
D����t��ʱ��AgCl(s)+Br-(aq) AgBr(s)+Cl-(aq)ƽ�ⳣ������816 AgBr(s)+Cl-(aq)ƽ�ⳣ������816 |
��ÿ��2�֣�
��1��������ϵ���¶ȣ���ֹ��Ļӷ���2�֣�
��2�����ˣ�����2�֣�
��3�� Br2+SO32-+H2O=2Br-+SO42-+2H+��2�֣�
��4�� CaO��Ca(OH)2��CaCO3��ȷ��Fe3+��Al3+������ȫ�ͷ�ֹ���������ܽ⣨��2�֣�
��5��AB��2�֣�
���������������1��Br2����SO2�ų��ܶ����������ӷ���ʹ�ñ�ˮ��������ϵ�¶ȣ���ֹ��������ʹ��Ӧ��ȫ��
��2���ɹ������̿�֪����������������Һ�壬Ӧ�ǹ��ˣ�������Ϊ���ܵ���Һ��ֵķ��룬Ӧ������
��3����Ϣ��м���Na2SO3��Na2SO3���л�ԭ�ԣ���Br2�������������ӷ���ʽΪ��Br2+SO32-+H2O=2Br-+SO42-+2H+
��4��Ŀ������ȡCaBr2��ͨ��������Һ��PHԼΪ8.0���ɳ�ȥ����Al3+��Fe3+��Ϊ�˷�ֹ�����ʵĽ��룬Ӧ���뺬CaԪ��������H+��Ӧ�����ʣ���CaO��Ca(OH)2��CaCO3��������Һ��PHԼΪ8.0ʱ��Al3+��Fe3+תΪAl(OH)3��Fe(OH)3��������ȥ�����Կ�����Һ��PHԼΪ8.0��Ŀ����ȷ��Fe3+��Al3+������ȫ�ͷ�ֹ���������ܽ⡣
��5��A������ͼ��c����������AgBr��Ksp=7��10-7��7��10-7= 4.9��10-13��С��AgCl��Ksp����ѡ����û�и���Cl?��Br?Ũ�ȣ����Բ�һ���Ȳ���AgBr�ij���������B����AgBr������Һ�м���NaBr���壬�����ܽ�ƽ���ƶ�����Һ��Ϊ������Һ����������c�㵽b�㣬����C��a�����ܽ�ƽ���������£�����a���Ӧ����AgBr�IJ�������Һ����ȷ��D����t��ʱ��AgCl(s)+Br-(aq) AgBr(s)+Cl-(aq)ƽ�ⳣ����=c��Cl?��/c(Br?)= c��Cl?��?c(Ag+)/c(Br?) ?c(Ag+)="Ksp(AgCl)/" Ksp(AgBr)= 4��l0-10/4.9��10-13��816����ȷ��
AgBr(s)+Cl-(aq)ƽ�ⳣ����=c��Cl?��/c(Br?)= c��Cl?��?c(Ag+)/c(Br?) ?c(Ag+)="Ksp(AgCl)/" Ksp(AgBr)= 4��l0-10/4.9��10-13��816����ȷ��
���㣺���⿼�黯ѧ���̵ķ������������������ӡ������ܽ�ƽ�⡣


 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����£���ijһԪ��BOH��HCl��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
| ʵ���� | HCl�����ʵ���Ũ�� ��mol��L-1�� | BOH�����ʵ���Ũ�� ��mol��L-1�� | �����Һ��pH |
| �� | 0.1 | 0.1 | pH=5 |
| �� | c | 0.2 | pH=7 |
| �� | 0.1 | 0.2 | pH>7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
���ʼ��仯�����ڹ�ũҵ������������Ҫ��Ӧ�á�
��1����֪25��ʱ��
SO2��g����2CO��g����2CO2��g����1/xSx��s�� ��H��akJ/mol
2COS��g����SO2��g����2CO2��g����3/xSx��s�� ��H��bkJ/mol��
��COS��g������CO��g����Sx��s����Ӧ���Ȼ�ѧ����ʽ�� ��
��2���ۻ�(As4S4)�ʹƻ�(As2S3)����ȡ�����Ҫ����ԭ�ϡ���֪As2S3��HNO3�����·�Ӧ��
As2S3+10H++ 10NO3?=2H3AsO4+3S+10NO2��+ 2H2O��������H3AsO4�����ʵ���
Ϊ0.6 mol��Ӧ��ת�Ƶ��ӵ���ĿΪ ��
��3��������ʵ���Ũ��Na2S��NaOH�����Һ�еμ�ϡ����������������H2S��HS?��S2?�ķֲ�������ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ�������μ���������Ĺ�ϵ����ͼ��ʾ�����Եμӹ���H2S������ݳ�����
��B��ʾ ��
�ڵμӹ����У���Һ����Ũ�ȴ�С��ϵ��ȷ���� (����ĸ)�� a��c(Na+)= c(H2S)+c(HS?)+2c(S2?)
a��c(Na+)= c(H2S)+c(HS?)+2c(S2?)
b��2c(Na+)=c(H2S)+c(HS?)+c(S2?)
c��c(Na+)=3[c(H2S)+c(HS?)+c(S2?)]
��NaHS��Һ�ʼ��ԣ����μ�������M��ʱ����Һ�и�����Ũ���ɴ�С��˳��Ϊ  ��4����ҵ������·ѭ����������������Ĺ�����������ͼ��ʾ��
��4����ҵ������·ѭ����������������Ĺ�����������ͼ��ʾ��
�� д����Ӧ���з�����Ӧ�Ļ�ѧ����ʽ�� ��
�� ������װ������ͼ��ʾ��д�������ĵ缫��Ӧʽ ����װ���з������ܷ�Ӧ�Ļ�ѧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��l�������£����ȡ0.1mol��L-1HA��Һ��0.1mol��L-1 NaOH��Һ�������ϣ���û��Һ��pH��8�����Һ����ˮ�������OH-Ũ����0.1mol��L-1 NaOH��Һ����ˮ�������OH-Ũ��֮��Ϊ ��
��2����ͬ�¶��£����������ᱵ����ֱ������ͬ����Ģ�0.1mol��L-1��������Һ
��0.1mol��L-1�Ȼ�����Һ������ˮ��0.1mol��L-1������Һ�У�Ba2+Ũ���ɴ�С��˳���� �����������д��
��3�������£���a mol��L-1�İ�ˮ��0.1mol��L-1������������ϣ�����Һ��c��NH4+����c(Cl-��ʱ���ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��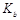 = mol��L-1��
= mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���������Ʒ�� NH4ClO4�ĺ������������вⶨ������װ����ͼ��ʾ(���Ⱥ������̶�װ������ȥ)��ʵ�鲽�����£�
���� 1������ͼ��ʾ��װ���������װ�������ԡ�
���� 2��ȷ��ȡ��Ʒa g (Լ 0.5 g)��������ƿ�У�����Լ 150 mL ˮ�ܽ⡣
���� 3��ȷ��ȡ 40.00 mL Լ 0.1 mol��L��1 H2SO4��Һ����ƿ�С�
���� 4������Һ©����������ƿ�м��� 20 mL 3 mol��L��1NaOH ��Һ��
���� 5������������������ƿ��ʣ��Լ 100 mL ��Һ��
���� 6��������й���ˮ��ϴ����װ�� 2��3 �Σ�ϴ��Һ������ƿ�С�
���� 7������ƿ�м������ָʾ������ c mol��L��1NaOH ����Һ�ζ����յ㣬���� NaOH ����Һ V1 mL��
���� 8����ʵ�鲽�� 1��7 �ظ� 2 �Ρ�
�ٲ��� 3�У�ȷ��ȡ 40.00 mL H2SO4��Һ�IJ��������� ��
�ڲ��� 1��7 �У�ȷ�����ɵİ���ϡ������ȫ���յ�ʵ�鲽���� (������)��
��Ϊ�����Ʒ�� NH4ClO4�ĺ��������貹���ʵ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧ����0.1000 mol��L-1������������Һ�ζ�δ֪Ũ�ȵ�ϡ���ᣬ������ɷֽ�Ϊ���¼�����
| A��ȡ20.00mL����������Һע��ྻ����ƿ�У�������2~3�η�̪��Һ |
| B���ñ�����������Һ��ϴ�ζ���2~3�� |
| C����ʢ�б�����������Һ��ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ |
| D��ȡ������������Һע���ʽ�ζ�������0���̶�����2~3mL |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС��Ϊ��̽������ĵ������������������ʵ�顣
ʵ��һ�����Ʋ��궨������Һ��Ũ��
ȡ����������250 mL 0.2 mol��L��1�Ĵ�����Һ����0.2 mol��L��1�Ĵ�����Һϡ�ͳ�����Ũ�ȵ���Һ������NaOH����Һ�����������Һ��Ũ�Ƚ��б궨���ش��������⣺
(1)����250 mL 0.2 mol��L��1������Һʱ��Ҫ�õ��IJ�����������Ͳ���ձ�����������__________________��______________��
(2)Ϊ�궨ij������Һ��ȷŨ�ȣ���0.200 0 mol��L��1��NaOH��Һ��20.00 mL������Һ���еζ������εζ�����NaOH��Һ��������£�
| ʵ����� | 1 | 2 | 3 | 4 |
| ����NaOH��Һ�����(mL) | 20.05 | 20.00 | 18.80 | 19.95 |
| ������ҺŨ��(mol��L��1) | 0.001 0 | 0.010 0 | 0.020 0 | 0.100 0 | 0.200 0 |
| pH | 3.88 | 3.38 | 3.23 | 2.88 | 2.73 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���ᾧ�����ɿɱ�ʾΪH2C2O4��xH2O��Ϊ�ⶨxֵ����������ʵ�顣
�ٳ�ȡm g���ᾧ�壬���100��0 mL��Һ��
��ȡ25��0 mL���������Һ������ƿ�У���������ϡH2SO4����Ũ��Ϊc mol��L��1 KMnO4��Һ�ζ����ζ�ʱ����������ӦΪ��2KMnO4��5H2C2O4��3H2SO4=K2SO4��10CO2����2MnSO4��8H2O
��ش��������⣺
��1��ʵ�����Ϊ������ȷŨ�ȵIJ�����Һ������Ҫ��ʵ��������Ҫ�У���ƽ(������)���ձ���ҩ��____________��____________��____________��
��2����ʵ����У��ζ�ʱKMnO4��ҺӦװ��________ʽ�ζ����У���ƿ��________
(���Ҫ���� ������Ҫ��)�μ�ָʾ����
��3���ڵζ������У�Ŀ��Ӧע��______________________��
��4�����ζ�ʱ���ζ�ǰ�����ζ����ֱ�ΪamL��bmL����˼����xֵΪ________��
��5������ȡ����aʱ���ӣ���ȡ����bʱ���ӣ�������xֵ________(�ƫ����ƫ С�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ͭ��п��ϡ������ɵ�ԭ���װ���У�����������1mol����ͨ��ʱ�������ϵ������仯�Ǣ�пƬ�ܽ���32��5�� ��пƬ������32��5�� ��ͭƬ������1��������ͭƬ������1mol����
| A���٢� | B���٢� | C���ڢ� | D���ڢ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com