
��l�������£����ȡ0.1mol��L-1HA��Һ��0.1mol��L-1 NaOH��Һ�������ϣ���û��Һ��pH��8�����Һ����ˮ�������OH-Ũ����0.1mol��L-1 NaOH��Һ����ˮ�������OH-Ũ��֮��Ϊ ��
��2����ͬ�¶��£����������ᱵ����ֱ������ͬ����Ģ�0.1mol��L-1��������Һ
��0.1mol��L-1�Ȼ�����Һ������ˮ��0.1mol��L-1������Һ�У�Ba2+Ũ���ɴ�С��˳���� �����������д��
��3�������£���a mol��L-1�İ�ˮ��0.1mol��L-1������������ϣ�����Һ��c��NH4+����c(Cl-��ʱ���ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��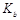 = mol��L-1��
= mol��L-1��
��6�֣�
��1��107��1(2��)
��2����>��>��>�� (2��)
��3��10-8/(a-0.1) (2��)
�������������
��1��0.1mol��L-1HA��Һ��0.1mol��L-1 NaOH��Һ�������ϣ��γ�NaA��Һ�����Һ��pH��8��Ϊǿ�������Σ����Һ����ˮ�������OH-Ũ�Ⱦ�����Һ��OH-Ũ��=Kw/c(H+) =10-7 mol��L-1 , 0.1mol��L-1 NaOH��Һ����ˮ�������OH-Ũ�ȵ�����Һ��H+Ũ��=10-13 mol��L-1����Ϊ107��1 ��
��2������BaSO4��s�� Ba2+��aq��+SO42-��aq���ij����ܽ�ƽ�⣬��0.1mol��L-1�Ȼ�����Һ���д�����Ba2+����0.1mol��L-1��������Һ�͢�0.1mol��L-1������Һ��ʹƽ�������ƶ��� ���Ԣ�>��>��>�� ��
Ba2+��aq��+SO42-��aq���ij����ܽ�ƽ�⣬��0.1mol��L-1�Ȼ�����Һ���д�����Ba2+����0.1mol��L-1��������Һ�͢�0.1mol��L-1������Һ��ʹƽ�������ƶ��� ���Ԣ�>��>��>�� ��
��3����Һ��c��NH4+����c(Cl-��=0.1mol��L-1/2����ϵ���غ��c��H+����c(OH-��=10-7 mol��L-1��NH3��H2O�ĵ��볣��Kb=
���㣺����������Һ�Ļ�ѧ���㡢�����ܽ�ƽ�⡢�����غ���ۺ�Ӧ�õ����֪ʶ��


 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������±ʯ(K2SO4��MgSO4��2CaSO4��2H2O)���ڡ�������ˮ�д��������ܽ�ƽ�⣺
K2SO4��MgSO4��2CaSO4��2H2O(s) 2Ca2����2K����Mg2����4SO42-��2H2O��Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�
2Ca2����2K����Mg2����4SO42-��2H2O��Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�
��1��������Ҫ�ɷ���________��CaSO4�Լ�δ����±ʯ��
��2���û�ѧƽ���ƶ�ԭ������Ca(OH)2��Һ���ܽ���±ʯ����K����ԭ��
��
��3�������ӡ������У��ȼ��� ��Һ��������Ȳ������ˣ��ټ��� ��Һ����ҺpH�����ԡ�
��4����ͬ�¶��£�K���Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ����ͼ����ͼ�ɵã������¶����ߣ�
�� ��
�� ��
���ܽ�����K����ƽ��Ũ������
��5�������Կ�����̼����Ϊ�ܽ��������ܽ������лᷢ����CaSO4(s)��CO32- CaCO3(s)��SO42-����֪298 Kʱ��Ksp(CaCO3)��2.80��10��9��Ksp(CaSO4)��4.90��10��5��������¶��¸÷�Ӧ��ƽ�ⳣ����K�� ��
CaCO3(s)��SO42-����֪298 Kʱ��Ksp(CaCO3)��2.80��10��9��Ksp(CaSO4)��4.90��10��5��������¶��¸÷�Ӧ��ƽ�ⳣ����K�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��12�֣�D��A��B��CΪ����ԭ��������������Ķ�����Ԫ�أ�A��B��Cͬ���ڣ�A��ԭ�Ӱ뾶��ͬ���������ģ�B��Dͬ���塣��֪DԪ�ص�һ�ֵ������ճ���������ˮ�����õ���������CԪ�صĵ��ʿ��Դ�A��B��Ԫ����ɵĻ������ˮ��Һ���û���BԪ�صĵ��ʡ�
��1��CԪ�������ڱ��е�λ�� ���� �塣
��2��A��BԪ���γɵij���������ˮ��Һ�� �ԣ�ԭ���ǣ������ӷ���ʽ��ʾ�� ��ʯī���缫���û������ˮ��Һ����������ӦʽΪ ��
��3��A��DԪ�ؿ����γɻ�����A2D2��д��A2D2��CO2��Ӧ�Ļ�ѧ����ʽ ����Ԫ�ط��ű�ʾ�����÷�Ӧ�л�ԭ���� ��
��4��BԪ�صĵ����ڲ�ͬ�������¿�����O2����һϵ�з�Ӧ���� B(s)+O2(g)=BO2(g)����H=��296.8kJ/mol��2BO2(g)+O2(g)  2BO3(g)����H=��196.6kJ/mol
2BO3(g)����H=��196.6kJ/mol
��1 mol BO3(g)����ȫ�ֽ��B��s������Ӧ�����е���ЧӦΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij�¶ȣ�t �棩ʱ��ˮ�����ӻ�ΪKw��1��10-13���������¶���pH��11�Ŀ�������Һa L��pH��1��ϡ����b L��ϣ����Ϻ���Һ�����С�仯���Բ��ƣ�����ͨ��������д���²�ͬ���ʱ������Һ������ȡ�
��1�������û����ҺΪ���ԣ���a��b��__________������Һ�и������ӵ�Ũ���ɴ�С������˳����
______________________________________________________________��
��2�������û����Һ��pH��2����a��b��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��20mL 0��0025mol��L-1 AgNO3��Һ�У�����5mL 0��01mol��L-1 KCl��Һ��ͨ�������ж��Ƿ���AgCl�������ɡ���֪Ksp��AgCl��=1��8��10-10 (��Ϻ���Һ�������Ϊ�������֮��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
CuSO4��Һ��K2C2O4��Һ��Ӧ���õ�һ����ɫ�ᾧˮ���ᄃ�塣ͨ������ʵ��ȷ���þ������ɣ�
�ٳ�ȡ0.1680g���壬���������H2SO4��Һ��ʹ��Ʒ�ܽ���������ˮ�����Ƚ��У���0.02000mol��L-1KMnO4��Һ�ζ����յ㣨��Һ��Ϊdz�Ϻ�ɫ��������20.00mL��
�ڽ��Ž���Һ��ּ��ȣ�ʹdz�Ϻ�ɫ��Ϊ��ɫ����ʱMnO��4ת��ΪMn2+���ͷų�O2��
����ȴ�����2g KI���壨������������Na2CO3����Һ��Ϊ��ɫ�����ɳ�����
����0.05000mol��L-1Na2S2O3��Һ�ζ������յ��ָʾ�����ζ����յ㣬����10.00mL��
��֪��2MnO��4+5H2C2O4+6H+==2Mn2++10CO2��+8H2O
2Cu2++4I��=2CuI��+I2
2Na2S2O3+I2=2NaI+Na2S4O6
��1��������з�����Ӧ�����ӷ���ʽΪ ��
��2��������м����ָʾ��Ϊ ��
��3��ͨ������д����ɫ����Ļ�ѧʽ��д��������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��14�֣�
�������ڹ�ҵ��ҽҩ���������Ź㷺����;����ͼ��ij��ȤС��ģ����Ʊ��������Ʒ�����Ƶ��������£�
��1����Ϣ�ʹ�ñ�ˮ��Ŀ���� ��
��2������II��III�������� , ��
��3����Ϣ��з�����Ӧ�����ӷ���ʽΪ ��
��4����ҵ����������ʹ���ʯ�Ƶ��廯���к�������Al3+��Fe3+���ʣ������������Լ� ���ѧʽ���������Һ��PHԼΪ8.0���ɳ�ȥ���ʣ�������Һ��PHԼΪ8.0��Ŀ����_______________________________________________________��
��5��t��ʱ����HBrͨ��AgNO3��Һ�����ɵ�AgBr��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪t��ʱAgCl��Ksp=4��l0-10������˵������ȷ���� �� ��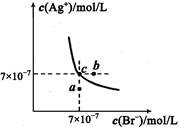
| A������Cl-��Br- �Ļ��Һ�еμ���������Һ��һ���Ȳ���AgBr�ij��� |
| B����AgBr������Һ�м���NaBr���壬��ʹ��Һ��c�㵽b�� |
| C��ͼ��a���Ӧ����AgBr�IJ�������Һ |
D����t��ʱ��AgCl(s)+Br-(aq) AgBr(s)+Cl-(aq)ƽ�ⳣ������816 AgBr(s)+Cl-(aq)ƽ�ⳣ������816 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ����0.10 mol��L��1 NaOH����Һ���вⶨ�����Ũ�ȵ�ʵ�顣ȡ20.00 mL�������������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶ�NaOH����Һ���еζ����ظ������ζ�����2��3�Σ���¼�������¡������������գ�
| ʵ���� | ������������(mL) | NaOH��Һ��Ũ��(mol��L��1) | �ζ����ʱ��NaOH��Һ��������(mL) |
| 1 | 20.00 | 0.10 | 24.18 |
| 2 | 20.00 | 0.10 | 23.06 |
| 3 | 20.00 | 0.10 | 22.96 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���и��仯������ԭ��صķ�Ӧ����
| A���ڿ����н���������Ѹ�������γɱ����� |
| B���Ӻ�ˮ��ͨ����ѧ�����õ�����þ |
| C�����ȵ���˿����ˮ�Ӵ��������������ɫ������ |
| D��п��ϡ���ᷴӦʱ������������CuSO4��Һ��ʹ��Ӧ�ӿ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com