
CuSO4��Һ��K2C2O4��Һ��Ӧ���õ�һ����ɫ�ᾧˮ���ᄃ�塣ͨ������ʵ��ȷ���þ������ɣ�
�ٳ�ȡ0.1680g���壬���������H2SO4��Һ��ʹ��Ʒ�ܽ���������ˮ�����Ƚ��У���0.02000mol��L-1KMnO4��Һ�ζ����յ㣨��Һ��Ϊdz�Ϻ�ɫ��������20.00mL��
�ڽ��Ž���Һ��ּ��ȣ�ʹdz�Ϻ�ɫ��Ϊ��ɫ����ʱMnO��4ת��ΪMn2+���ͷų�O2��
����ȴ�����2g KI���壨������������Na2CO3����Һ��Ϊ��ɫ�����ɳ�����
����0.05000mol��L-1Na2S2O3��Һ�ζ������յ��ָʾ�����ζ����յ㣬����10.00mL��
��֪��2MnO��4+5H2C2O4+6H+==2Mn2++10CO2��+8H2O
2Cu2++4I��=2CuI��+I2
2Na2S2O3+I2=2NaI+Na2S4O6
��1��������з�����Ӧ�����ӷ���ʽΪ ��
��2��������м����ָʾ��Ϊ ��
��3��ͨ������д����ɫ����Ļ�ѧʽ��д��������̣���
��1��4MnO4- +12H+=4Mn2++5O2��+6H2O
��2��������Һ
��3��n(C2O42-)=0.02000��20.00��10-3��5/2=1.00��10-3mol
n(Cu2+)=0.05000��10.00��10-3=5.00��10-4mol
���ݵ���غ��֪�����廹����������K+��
n(K+)=2��1.00��10-3-2��5.00��10-4=1.00��10-3mol
�ʾ���Ļ�ѧʽΪK2Cu(C2O4)2��xH2O
m(H2O)=0.1680-5.00��10-4��318=0.009g
n(H2O)=0.009/18=5.00��10-4mol
�ʾ���Ļ�ѧʽΪK2Cu(C2O4)2��H2O
�����������������ⶨ������ɵ��ۺϼ����⣬(2)�еⵥ�ʲ�������ɵķ�Ӧ�������õ�����Ϊ��Ӧ�Ƿ���ȫ�����ݣ���3�����ݵڢٲ���Ӧ���Լ����C2O42-������n(C2O42-)=0.02000��20.00��10-3��5/2=1.00��10-3mol
�ڢ��dz�ȥ��Һ�ж���ĸ��������Һ���ڢۢܿ��Լ����ͭ���ӵ���
n(Cu2+)=0.05000��10.00��10-3=5.00��10-4mol
���ݵ���غ��֪�����廹����������K+��
n(K+)=2��1.00��10-3-2��5.00��10-4=1.00��10-3mol
�ʾ���Ļ�ѧʽΪK2Cu(C2O4)2��xH2O
Ȼ���ٸ���������������ᾧˮ������m(H2O)=0.1680-5.00��10-4��318=0.009g
n(H2O)=0.009/18=5.00��10-4mol
�ʾ���Ļ�ѧʽΪK2Cu(C2O4)2��H2O
���㣺�����Թ�ҵ�ⶨ������ɶ���Ƶļ����⣬�漰���ӷ���ʽ��д���ζ�ָʾ��ѡ�����غ������й����⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(һ)��1������Ҳ��һ�����ȼ�ϣ�������ȫȼ��ʱ��Ч�ʽ��Ͳ�������ж����������Ⱦ��
��֪�� CH4(g) + 2O2(g) �� CO2(g) + 2H2O(l) ��H1���D890.3 kJ/mol
2CO (g) + O2(g) �� 2CO2(g) ��H2���D566.0 kJ/mol
����鲻��ȫȼ������һ����̼��Һ̬ˮʱ����Ч��ֻ����ȫȼ��ʱ��________��������������1λС������
��2������ȼ�ϵ�ؿ����������������ʡ���ͼ�����ü���ȼ�ϵ�ص��50 mL 2 mol/L���Ȼ�ͭ��Һ��װ��ʾ��ͼ��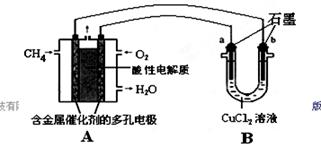
��ش�
�ټ���ȼ�ϵ�صĸ�����Ӧʽ��________��
�ڵ���·����0.1 mol����ͨ��ʱ��________���a����b����������________g��
�������±��Ǽ���������ʵĵ���ƽ�ⳣ�������ܵ���ʵ�
�ܶȻ�Ksp (25��)��
| ����� | ƽ�ⷽ��ʽ | ƽ�ⳣ��K | Ksp |
| CH3COOH | CH3COOH CH3COO-��H+ CH3COO-��H+ | 1.76��10-5 | |
| H2CO3 | H2CO3 H+��HCO3- H+��HCO3-HCO3-  H+��CO32- H+��CO32- | K1��4.31��10-7 K2��5.61��10-11 | |
| C6H5OH | C6H5OH  C6H5O-��H+ C6H5O-��H+ | 1.1��10-10 | |
| H3PO4 | H3PO4 H+��H2PO4- H+��H2PO4-H2PO4-  H+��HPO32- H+��HPO32-HPO42-  H+��PO43- H+��PO43- | K1��7.52��10-3 K2��6.23��10-8 K3��2.20��10-13 | |
| NH3��H2O | NH3��H2O NH4+��OH- NH4+��OH- | 1.76��10-5 | |
| BaSO4 | BaSO4 Ba2+��SO42- Ba2+��SO42- | | 1.07��10-10 |
| BaCO3 | BaCO3 Ba2+��CO32- Ba2+��CO32- | | 2.58��10-9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��15�֣���ҵ����ƽ�VOSO4�е�K2SO4��SiO2���ʳ�ȥ�����յõ�V2O5���������£�
��ش��������⣺
��1����������÷����ijɷ��� ��д��ѧʽ��������I������ ��
��2������ڡ��۵ı仯���̿ɼ�Ϊ����ʽR��ʾVO2+��HA��ʾ�л���ȡ������
R2(SO4)n (ˮ��)+ 2nHA���л��㣩 2RAn���л��㣩 + nH2SO4 (ˮ��)
2RAn���л��㣩 + nH2SO4 (ˮ��)
������ȡʱ��������������ԭ���� ��
����X�Լ�Ϊ ��
��3���ܵ����ӷ���ʽΪ ��
��4��25��ʱ��ȡ����������������õ��������ʺ���ҺpH֮���ϵ���±���
| pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��17�֣������ҹ���ҵ��ˮƽ�IJ��Ϸ�չ�����ˮ��������Ⱦ�����Ϊ��Ҫ���⡣
��1������β���Ĵ����ŷ�����ɿ�����Ⱦ����Ҫ����֮һ����չȼ�ϵ������������Ч�ؽ���������⡣ֱ�Ӽ״�ȼ�ϵ��(DMFC)��������к��������ת��Ч�ʱ���ȼ��Ҫ��2��3������ؽṹ��ͼ��ʾ��c��ͨ�������ΪΪ______�����·�е��Ӵ�______��______(�A����B��)�ƶ���д����ظ����ĵ缫��Ӧ����ʽ 
��2����ҵ��ˮ�г�����һ������Cr2O72����������༰��̬ϵͳ�����ܴ�����ⷨ�Ǵ�������Ⱦ�ij��÷������÷���Fe���缫��⺬Cr2O72�������Է�ˮ�����ʱ�����������д����������ɣ�������Cr(OH)3��Fe(0H)3������
�ٷ�Ӧ�У�1molCr2O72����ȫ����Cr(OH)3���������·ͨ�����ӵ����ʵ���Ϊ_________ mol��
�ڳ����£�Cr(OH)3���ܶȻ� ����Cr3��Ũ��С��10
����Cr3��Ũ��С��10 mol
mol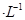 ʱ����Ϊ��ȫ�����������ȫ�����Һ��pH=6�������Һ���˺�Ϊ___________����ܡ���ֱ���ŷš�
ʱ����Ϊ��ȫ�����������ȫ�����Һ��pH=6�������Һ���˺�Ϊ___________����ܡ���ֱ���ŷš�
��3��������ˮ������ˮ�帻Ӫ��������NH4Cl��Һ�м�������NaOH���壬��Һ�� ________���������С�����䡱����25
________���������С�����䡱����25 ʱ��NH3?H2O�ĵ���ƽ�ⳣ��
ʱ��NH3?H2O�ĵ���ƽ�ⳣ�� �����¶���,1mol
�����¶���,1mol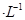 ��NH4Cl��Һ��
��NH4Cl��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��l�������£����ȡ0.1mol��L-1HA��Һ��0.1mol��L-1 NaOH��Һ�������ϣ���û��Һ��pH��8�����Һ����ˮ�������OH-Ũ����0.1mol��L-1 NaOH��Һ����ˮ�������OH-Ũ��֮��Ϊ ��
��2����ͬ�¶��£����������ᱵ����ֱ������ͬ����Ģ�0.1mol��L-1��������Һ
��0.1mol��L-1�Ȼ�����Һ������ˮ��0.1mol��L-1������Һ�У�Ba2+Ũ���ɴ�С��˳���� �����������д��
��3�������£���a mol��L-1�İ�ˮ��0.1mol��L-1������������ϣ�����Һ��c��NH4+����c(Cl-��ʱ���ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��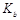 = mol��L-1��
= mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʳ�ð״����ɴ����봿ˮ���ƶ��ɣ����к͵ζ��ķ���ȷ�ⶨ���д�������ʵ���Ũ�ȡ�ʵ�鲽�裺������500mLŨ��ԼΪ0��1mol��L-1��NaOH��Һ������KHC8H4O4����Һȷ�ⶨ��NaOH��Һ��Ũ�ȣ�������֪ȷŨ�ȵ�NaOH��Һ�ⶨ�����Ũ�ȡ�
��1�����������NaOH�������ڴ��ձ��У�����500mL����ˮ�������ܽ⡣�����Ʋ��� ������С������С�����
��2������ʱNaOH�ڿ����м�����ˮ���������õ�NaOH��ҺŨ��ͨ����Ԥ�� ���С���������Dz���ֱ�����������Һ��ԭ��
��3�����İ״װ�װ�����Ậ��ԼΪ6g/100mL����������ʵ�Ũ��ԼΪ mol��L-1���ζ�ǰҪ�Ƚ��״�ϡ��10����ϡ�Ͱ״�ʱ��Ҫ��������100mL����ƿ���ձ����������� ��ͷ�ιܡ� ��
��4��ȷ��ȡϡ�ͺ�İ״�20��00mL������250mL��ƿ�У�����30mL����ˮ���ٵμӷ�ָ̪ʾ����������NaOH����Һ�ζ��� ��Ϊ�յ㡣
��5��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡ�״������Ϊ20��00mL��NaOH��ҺŨ��Ϊc mo1/L������ʵ ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 25��02 | 24��22 | 24��18 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧ����0.1000 mol��L-1������������Һ�ζ�δ֪Ũ�ȵ�ϡ���ᣬ������ɷֽ�Ϊ���¼�����
| A��ȡ20.00mL����������Һע��ྻ����ƿ�У�������2~3�η�̪��Һ |
| B���ñ�����������Һ��ϴ�ζ���2~3�� |
| C����ʢ�б�����������Һ��ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ |
| D��ȡ������������Һע���ʽ�ζ�������0���̶�����2~3mL |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ�������ָʾ��������д���пհף�
(1)�ñ�����ζ����������������Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע�� ��ֱ������һ���������Һ��ɫ�ɻ�ɫ��Ϊ��ɫ���� Ϊֹ��
(2)���в����п���ʹ��������������Һ��Ũ��ֵƫ�͵��� ��
| A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע������� |
| B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����δ���� |
| C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ |
| D����ȡ�������ʱ����ʼ���Ӷ������ζ��������Ӷ��� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ͼ��һ��Ӧ�ù㷺��﮵�أ�LiPF6�ǵ���ʣ�SO(CH3)2���ܼ�����Ӧԭ����4Li+FeS2��Fe+2Li2S������˵������ȷ����
| A��������ˮ����SO(CH3)2���ܼ� | B�������ƶ���������a������b�� |
| C����װ�ý���ѧ��ת��Ϊ���� | D��b����Ӧʽ��FeS2��4Li+��4e-��Fe��2Li2S |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com