
����Ŀ�����������Ǽ�������������Ч�ɷ֡�ʵ�����Ʊ��������Ƶ�װ������ͼ����ʾ��

��1��װ��C�����ɸ����ʵ����ʵ�������Һ���¶���ʱ��ı仯��ͼ����ʾ��t1���Ӻ���������Ҫ��Ӧ�Ļ�ѧ����ʽΪ________________________��
��2�����Ҫ����NaClO3�����ɣ����Բ�ȡ�ķ�����_________��___________(������)��
��3����װ��C�е���Һ�õ�����������Ʒ������ô���������Ʒ�л��е�����ΪNaClO3��NaCl�е�һ�֡���ȡ2.0225 g��Ʒ����ƿ�У���ˮʹ����ȫ�ܽ⣬����Һ�м���200 mL 0.60 mol��L��1��FeSO4��Һ(����)����ַ�Ӧ��������Һ�еμ�0.50 mol��L��1��KMnO4��Һ��ǡ����ȫ��Ӧʱ����KMnO4��Һ20.00 mL���������Ʒ��NaClO����������____________��д�������������
��֪��H����ClO����Fe2����Cl����Fe3����H2O
H����ClO3-��Fe2����Cl����Fe3����H2O
H����MnO4-��Fe2����Mn2����Fe3����H2O�����Ϸ�Ӧ��δ��ƽ��
���𰸡�3Cl2��6NaOH��NaClO3��5NaCl��3H2O �����μ����� ��װ��C���ڱ�ˮ�� ����KMnO4���ʵ�����0.5 mol��L��1��0.02 L��0.01 mol ����KMnO4��Ӧ��Fe2�����ʵ�����0.01 mol��5��0.05 mol��Fe2�������ʵ�����0.60 mol��L��1��0.2 L��0.12 mol������Ʒ��Ӧ��Fe2�����ʵ�����0.12 mol��0.05 mol��0.07 mol ���������ۣ�2.0225 g��ƷȫΪNaClO��2n(NaClO)��n(Fe2��)��n(Fe2��)��2��(2.0225 g��74.5 g��mol��1)��0.0543 mol��2.0225 g��ƷȫΪNaClO3��6n(NaClO3)��n(Fe2��)��n(Fe2��)��6��(2.0225 g��106.5 g��mol��1)��0.1139 mol��2.0225 g��ƷȫΪNaCl��������Fe2��������Ʒ�к�������ΪNaClO3 ���з�����ã�74.5n(NaClO)��106.5n(NaClO3)��2.0225 g �٣�2n(NaClO)��6n(NaClO3)��0.07 mol �ڣ��ⷽ����ã�n(NaClO)��0.02 mol�� ��(NaClO)��(0.02mol��74.5 g��mol��1��2.0225g)��100%=73.67%
��������
����ͼʾ���̷������ʵ��Ʊ������롢�ᴿ�Ĺ��̣������й����ʵ����ʵ���������ؼ��㡣
(1)��ͼ��֪��Ũ�����������ط�Ӧ����Cl2�����������Ȼ�����Һ��ȥδ��Ӧ��HCl��������Cװ�������������Ʒ�Ӧ����ͼ�ҿ�֪����Ӧ���й����У��¶����ߣ�t1���Ӻ�������NaClO3����t1���Ӻ���������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��3Cl2��6NaOH��NaClO3��5NaCl��3H2O��
�ʴ�Ϊ��3Cl2��6NaOH��NaClO3��5NaCl��3H2O��
(2)��ͼ�ҿ�֪���¶�Խ�ߣ�NaClO3Խ�������ɣ�������Ҫ��СNaClO3�������ʣ����Խ��ͷ�Ӧװ�õ��¶Ȼ����HCl����μ��ٶȣ��Ӷ�����NaClO�IJ��ʣ�
�ʴ�Ϊ�������μ����ᣬ��װ��C���ڱ�ˮ�У�
(3)������֪������ƽ�ɵõ����ӷ���ʽΪ��8H����MnO4-��5Fe2��=Mn2����5Fe3����4H2O������KMnO4���ʵ�����0.5mol��L��1��0.02L��0.01 mol����KMnO4��Ӧ��Fe2�����ʵ�����0.01 mol��5��0.05mol������Ʋ�������������n(Fe2+)=0.60mol��L��1��0.2L-0.05mol��0.12 mol-0.05mol=0.07mol���ٸ��ݼ������ۣ���2.0225 g��ƷȫΪNaClO��2n(NaClO)��n(Fe2��)��n(Fe2��)��2��(2.0225g��74.5g��mol��1)��0.0543mol��2.0225g��ƷȫΪNaClO3��6n(NaClO3)��n(Fe2��)��n(Fe2��)��6��(2.0225g��106.5g��mol��1)��0.1139 mol��2.0225 g��ƷȫΪNaCl��������Fe2��������Ʒ��һ����������ΪNaClO3��ʣ��ɸ������ӷ���ʽ�ĵ�����ϵ�з����飺74.5n(NaClO)��106.5n(NaClO3)��2.0225g �٣����������Ϊ2.0225g����
2n(NaClO)��6n(NaClO3)��0.07mol��[��n(NaClO)��n(NaClO3)��ʾn(Fe2+)]���ⷽ����ã�n(NaClO)��0.02mol����(NaClO)��(0.02mol��74.5g��mol��1��2.0225g)��100%=73.67%��
�ʴ�Ϊ������KMnO4���ʵ�����0.5mol��L��1��0.02L��0.01mol ����KMnO4��Ӧ��Fe2�����ʵ�����0.01mol��5��0.05mol��Fe2�������ʵ�����0.60mol��L��1��0.2L��0.12mol������Ʒ��Ӧ��Fe2�����ʵ�����0.12mol��0.05mol��0.07mol ���������ۣ�2.0225 g��ƷȫΪNaClO��2n(NaClO)��n(Fe2��)��n(Fe2��)��2��(2.0225 g��74.5 g��mol��1)��0.0543 mol��2.0225 g��ƷȫΪNaClO3��6n(NaClO3)��n(Fe2��)��n(Fe2��)��6��(2.0225g��106.5 g��mol��1)��0.1139 mol��2.0225g��ƷȫΪNaCl��������Fe2��������Ʒ�к�������ΪNaClO3 ���з�����ã�74.5n(NaClO)��106.5n(NaClO3)��2.0225g�٣�2n(NaClO)��6n(NaClO3)��0.07 mol�ڣ��ⷽ����ã�n(NaClO)��0.02 mol����(NaClO)��(0.02mol��74.5 g��mol��1��2.0225g)��100%=73.67%��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��EPR��(���ұ�ϩ)���̲��Ͼ�̼����(���PC)��һ�ֺϳ�·�����£�

��֪�����뺬�ǻ��Ļ�����ɷ���������������Ӧ��RCOOR��+R��OH![]() RCOOR��+R��OH
RCOOR��+R��OH
��ش�
(1)D��������������_______��
(2)EPR�Ľṹ��ʽ_______��
(3)����˵����ȷ����_______(����ĸ)��
a.��Ӧ����ԭ��������Ϊ100%
b.��Ӧ��Ϊȡ����Ӧ
c.1mol F����������Na��Ӧ����������22.4L H2(��״����)
(4)��Ӧ���Ļ�ѧ����ʽ��_______��
(5)��Ӧ������Eʱ��������ָ����������һ�ַ���ʽΪC9H12O2�ĸ�����M����ṹ��ʽΪ_______��
(6)��Ӧ���Ļ�ѧ����ʽ��_______��
(7)��֪��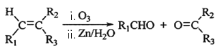 (R1��R2��R3��������)д����DΪԭ�Ϻϳɼ�����[HOOC(CH2)4COOH]�ĺϳ�·�ߣ����Լ���ѡ���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ����_______________________��
(R1��R2��R3��������)д����DΪԭ�Ϻϳɼ�����[HOOC(CH2)4COOH]�ĺϳ�·�ߣ����Լ���ѡ���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ����_______________________��
ʾ����CH3CH2OH![]() CH2=CH2
CH2=CH2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(���THC)���Ǵ����е���Ҫ����������ʣ���ṹ��ͼ�������й�THC��˵������ȷ����

A. THC������ˮ
B. 1mol THC�����뺬3mol�嵥�ʵ���ˮ������Ӧ
C. THC��FeCl3��Һ�ܷ�����ɫ��Ӧ
D. THC��������������Һ��̼������Һ��̼��������Һ������ѧ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧ����ʽ�У�������ȷ���ﷴӦ��ɫ�仯��ԭ�����
A. ͭ���ÿ����б��������ɫ���壺2Cu��O2��CO2��H2O�TCu2(OH)2CO3
B. ij�ֻ�������������N2O4���ݳ���������ɫ���壺N2O4![]() 2NO2
2NO2
C. FeSO4��7H2O�ڿ����о��ñ�ƣ�2FeSO4��7H2O![]() Fe2O3��SO2����SO3����14H2O
Fe2O3��SO2����SO3����14H2O
D. SO2ͨ��KMnO4��Һ�У���Һ��ɫ����ȥ��5SO2��2KMnO4��2H2O�TK2SO4��2MnSO4ʮ2H2SO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���Ҵ����ϴ���FeBr3��Һ���õ���ı���Һ����ˮFeCl3����������ܴﵽ��Ӧʵ��Ŀ�ĵ���
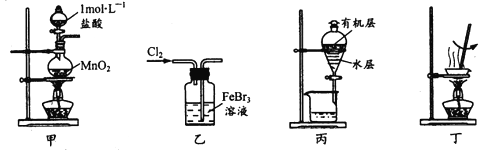
A. ��װ�ü���ȡ����
B. ��װ����ʹBr�� ȫ��ת��Ϊ�嵥��
C. ��װ�ñ���Һʱ�ȴ��¿ڷų�ˮ�㣬�ٴ��Ͽڵ����л���
D. ��װ�ö�����Һ���ˮ���������ɣ��������Ƶ���ˮFeCl3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�������ݣ�
�� �� | �۵�/�� | �е�/�� | �ܶ�/g��cm��3 |
�� �� | ��114 | 78.4 | 0.79 |
�� �� | 16.6 | 117.9 | 1.05 |
�������� | ��83.6 | 77.5 | 0.900 |
ŨH2SO4 | 338 | 1.84 |



ʵ������ȡ������������Ҫװ������ͼI��ʾ����Ҫ����Ϊ������30mL�Ĵ��Թ��а������2��3��2�ı�������Ũ���ᡢ�Ҵ�������Ļ��Һ���ڰ���ͼI����װ�ã�ʹ����������������ͨ��ʢ��10mL����Na2CO3��Һ��(����2�η�̪��Һ)�Թ��У���С������Թ��еĻ��Һ���ܴ�С�Թ����ռ�Լ2mL����ʱֹͣ���ȣ�����С�Թܲ�������Ȼ���ã��ݷ����������������������ش��������⣺
��1��������У�������һ�����Ļ��Һ�IJ�����_____________________________��
��2��д����ʵ����ȡ���������Ļ�ѧ����ʽ_________________________________��ŨH2SO4�������� _______________________��
��3��������У���С������Թ��еĻ��Һ����ԭ��_________________________��
��4����������۲쵽��������___________________________________________________
��5��������У���������������ķ�����_________________________________��
��6��Ϊ������������IJ��ʣ��ס�����λͬѧ�ֱ����������ͼ�ס��ҵ�װ��(��ͬѧ����Ӧ�����ȴ�����ñ���Na2CO3��Һ��ȡԲ����ƿ�в���)������Ϊ����װ�ø�������Ϊʲô��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ը�����FeO��Cr2O3��Al2O3��SiO2�ȣ�Ϊԭ���Ʊ���������ص�ʵ�鲽����ͼ��

�ش��������⣺
��1�������ڡ���װ����ͼ������W�IJ��ʿ�����___����������մɡ�����������FeO��Cr2O3��KClO3��Na2CO3������Ӧ������Fe2O3��KCl��Na2CrO4��CO2�Ļ�ѧ����ʽΪ____��

��2�����ں�Ĺ����к�Na2CrO4��Fe2O3��Na2SiO3��NaAlO2��KCl��NaFeO2�ȣ�����ٵľ��岽��Ϊˮ�������ˣ���pHΪ7~8��������а�Сʱ�����ȹ��ˡ���һ�ι��������е���Ҫ�ɷ�Ϊ_____��
��3�������������ᣬ�����ϡ����ʱ������Ӧ�����ӷ���ʽΪ____��
��4���ɲ���ۿ�֪���ܽ�ȣ�Na2Cr2O7____���>����<���T�T����K2Cr2O7��
��5������ܰ����ľ��������___��___���˲�ϴ�ӣ�������õ�K2Cr2O7���塣���й����ʵ��ܽ��������ͼ��ʾ��

��6��������������ֹ��������м���һ��ˮ�������ƾ���ĥ�����õĹ�������������������____��
��7���������ط������ⶨK[Cr��C2O4��2]��nH2O��Ʒ�����ᾧˮ��������Ʒ���ȵ�80��Cʱ��ʧ��ȫ���ᾧˮ��ʧ��16.8%��K[Cr��C2O4��2]��nH2O������n=____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ھ����˵��һ����ȷ����(����)
A. �ڢ�A������Ԫ�����A��Ԫ�����γɵĻ������ڹ�̬ʱΪ���Ӿ��壬�������������������з�ʽ��ͬ
B. �����д��������Ӿͱض����������ӣ����������Ӿͱض�����������
C. ���Ӿ�����ֻ�������Ӽ������Ӿ��塢ԭ�Ӿ����бض����й��ۼ�
D. C60����(��ṹģ����ͼ)��ÿ��C60������Χ������������ҵȾ����C60������12��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������A(����ʽΪC6H6O)��һ���л�����ԭ�ϣ��ڿ������ױ�������Ϊ�ۺ�ɫ��A ���й�ת����Ӧ����(���ַ�Ӧ������ȥ)��

��֪�� ��R��ʾ��������
��R��ʾ��������
(1)д��A �����ƣ�________������A���õ��Լ���__________��
(2)д��G�к��������ŵ����ƣ�_________��_________��
(3) C�ķ���ʽ_________ ��ΪD�Ľṹ��ʽΪ____________��
(4)F��D��Ϊͬ���칹�塣д����ӦE��F�Ļ�ѧ����ʽ��___________��
(5)ij��������E��ͬ���칹�壬�ҷ�����ֻ�����ֲ�ͬ��ѧ�������⡣д���û�����Ľṹ��ʽ��_______(��дһ��)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com