

| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
| Y | M������2�ԳɶԵ��� |
| Z | Z��Yͬ���ڣ�Z�ĵ縺�Դ���Y |
| W | W��һ�ֺ��ص�������Ϊ63��������Ϊ34 |


 ��
�� ��
�� ���ṹʽΪS=C=S������2���Ҽ�������Cl�ĵ縺�Ա�Sǿ������H-Y��H-Z���ֹ��ۼ��У����ļ��Խ�ǿ����H-Z��
���ṹʽΪS=C=S������2���Ҽ�������Cl�ĵ縺�Ա�Sǿ������H-Y��H-Z���ֹ��ۼ��У����ļ��Խ�ǿ����H-Z�� ��2��H-Z��
��2��H-Z��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?����һģ������ѧһ���ʽṹ�����ʡ�
��2013?����һģ������ѧһ���ʽṹ�����ʡ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| (b+d) |
| 2 |
| (c+e) |
| 2 |

| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1���ڵ�3�����У��û��������������ǿ��Ԫ�ص�Ԫ�ط���Ϊ
��1���ڵ�3�����У��û��������������ǿ��Ԫ�ص�Ԫ�ط���Ϊ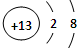
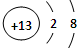
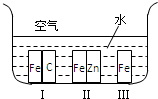
 C��g����������������ʱ���ı�����һ��������������C�����ʣ���ӿ족�����������䡱����?a������
C��g����������������ʱ���ı�����һ��������������C�����ʣ���ӿ족�����������䡱����?a�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
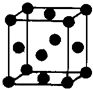 ��2012?�人ģ�⣩[��ѧһѡ�� 3�����ʽṹ������]��U��V��W��X��Y��Z ����ǰ������Ԫ�أ�ԭ���������������������Ϣ���±���
��2012?�人ģ�⣩[��ѧһѡ�� 3�����ʽṹ������]��U��V��W��X��Y��Z ����ǰ������Ԫ�أ�ԭ���������������������Ϣ���±���| Ԫ�ر�� | �����Ϣ�� |
| U | ���������������������ֱ�����ԭ��������� |
| V | ��̬ʱ�����ӷֲ��������ܼ��ϣ��Ҹ��ܼ��е�������� |
| W | ��̬ʱ��2p ������ڰ����״̬ |
| X | ��WԪ�ش���ͬһ���ڣ���X�ĵ�һ������С��W�ĵ�һ���� |
| Y | �ǵ�������Ԫ����δ�ɶԵ���������Ԫ�� |
| Z | Z ��һ�ֺ��ص�������Ϊ65��������Ϊ 36 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com