
����Ŀ��100��ʱ����1 L���º��ݵ��ܱ������У�ͨ��0.1molN2O4��������Ӧ��N2O4(g)![]() 2NO2(g)��H=+57.0kJ��mol-1��NO2��N2O4��Ũ����ʱ��仯�����ͼ��ʾ��
2NO2(g)��H=+57.0kJ��mol-1��NO2��N2O4��Ũ����ʱ��仯�����ͼ��ʾ��

��.��1����0~60 s�ڣ���N2O4��ʾ��ƽ����Ӧ����Ϊ____mol��L-1��s-1��
��2����Ӧ���е�100sʱ����ֻ��һ�����������仯����仯������������____��
A�������¶� B��ͨ�뺤��
C�����������г���N2O4 D�����������ݻ�
��3����֪�� N2(g)+2O2(g)=2NO2(g) ��H=+67.2kJ��mol-1��N2H4(g)+O2(g)=N2(g)+2H2O(g) ��H=-534.7kJ��mol-1��N2O4(g)=2NO2(g) ��H=+57.0kJ��mol-1����2N2H4(g)+N2O4(g)=3N2(g)+4H2O(g) ��H=____kJ��mol-1��
��.���ݻ�Ϊ2 L���ܱ�������ͨ��һ������CO��H2O��������Ӧ��CO(g)+H2O(g)![]() H2(g)+CO2(g)��
H2(g)+CO2(g)��
��4�����������������䣺
������ƽ����ϵ����ͨ��0.20molH2O(g)��ƽ�⽫___(����������������������������)�ƶ����ﵽ�µ�ƽ��״̬��H2O(g)�����������____(�������������С������������)��
����VL�ܱ�������ͨ��10molCO��10molH2O(g)����������Ӧ����T���ﵽƽ�⣬Ȼ���ٳ�ȥˮ����(��ˮ����ʱ�������ɷֵ����ʵ�������)�����������ȼ�գ���÷ų�������Ϊ2842kJ(��֪CO��ȼ����Ϊ283kJ��mol-1��H2��ȼ����Ϊ286kJ��mol-1)����T��ƽ�ⳣ��K=____������ȷ��С�������λ��
���𰸡�1��10-3 A -1079.6 ���� ��� 0.44
��������
��ͼ��֪����ʼʱc��N2O4��=0.1mol/L��60��ʱ��Ӧ����ƽ��״̬ʱc��N2O4��=0.04mol/L��c��NO2��=0.12mol/L��
��1������v=��c/��t���㷴Ӧ���ʣ�
(2).����ƽ���ƶ��������ı仯��������жϣ�
��3�����ݸ�˹���ɼ������Ӧ�ȣ�д���Ȼ�ѧ����ʽ
��4��CO��H2�����ʵ�����Ϊ10mol������ȼ�շų����������CO��H2���Ե����ʵ�������������ʽ�����ƽ��ʱ����ֵ����ʵ���������ƽ�ⳣ�����㣮
����ͼ��֪����ʼʱc(N2O4)=0.1mol/L��60��ʱ��Ӧ����ƽ��״̬ʱc(N2O4)=0.04mol/L��c(NO2)=0.12mol/L��(1)����v=��c/��t����N2O4��ʾ��ƽ����Ӧ����Ϊ��0.1mol/L0.04mol/L��![]() 60s=1��10-3mol/(Ls)��
60s=1��10-3mol/(Ls)��
�ʴ�ΪΪ��1��103��
��2��A. �÷�Ӧ�����ȷ�Ӧ�������¶ȣ�ƽ�����ƣ�c(NO2)��С��c(N2O4)������ͼ���������Aѡ��
B. ���º���ͨ�뺤����ƽ�ⲻ�ƶ��������Ũ�Ȳ��䣬��ͼ������B��ѡ��
C�����������г���N2O4��100sʱN2O4Ũ������c(NO2)Ҳ����N2O4Ũ������Ӧ�Ͽ�����ͼ������C��ѡ��
D. �����������������ֵ�Ũ�ȶ���С����ͼ������D��ѡ��
��ѡA��
��3����N2(g)+2O2(g)=2NO2(g) ��H=+67.2kJ��mol-1.
��N2H4(g)+O2(g)=N2(g)+2H2O(g) ��H=-534.7kJ��mol-1��
��N2O4(g)=2NO2(g) ��H=+57.0kJ��mol-1
���ݸ�˹����,����2��+�ۿ�д���Ȼ�ѧ����ʽ2N2H4(g)+N2O4(g)=3N2(g)+4H2O(g) ��H=534.7kJmol1��267.2kJmol1+57.0kJmol1=1079.6kJmol1��
�ʴ�Ϊ��1079.6.
���ٱ��������������䣬��ƽ����ϵ����ͨ��0.20 mol H![]() O(g)����ԭƽ�����ƽ�������ƶ���������ͨ����H
O(g)����ԭƽ�����ƽ�������ƶ���������ͨ����H![]() O(g)���ʴﵽ�µ�ƽ��״̬��H
O(g)���ʴﵽ�µ�ƽ��״̬��H![]() O(g)������������
O(g)������������
���ɷ���ʽCO(g)+H2O(g)H2(g)+CO2(g)��֪��1molCO��Ӧ����1molH2,��ʼͨ��10molCO��ƽ��ʱCO��H2�����ʵ���֮��Ϊ10mol
��CO��H2���ʵ���Ϊx��y����![]() ��
��
��ã�![]() ��
��
��������ʽ�����ƽ��ʱ����ֵ����ʵ�����
CO(g)+H2O(g)H2(g)+CO2(g)��
��ʼ��10mol10mol00
ת����4mol4mol4mol4mol
ƽ�⣺6mol6mol4mol4mol����T��ʱ��Ӧ��ƽ�ⳣ��Ϊ��K=![]() =
=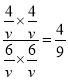 =0.44
=0.44
�ʴ�Ϊ��0.44



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���и�������һ������ָ�������д���������ǣ� ��
A����c(H+)=10-10 mol/L����Һ�� Al3+ ��NH![]() ��Cl�� ��NO
��Cl�� ��NO![]()
B��pHֵΪ13����Һ K+ ��SO![]() ��Na+��S2-
��Na+��S2-
C��ˮ���������c(H+)=10��12mol/L����Һ K+��NH4+��Cl����ClO-
D�����ȳʺ�ɫ����Һ�� Fe3+��Na+ ��SO42-��CO![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CO2��������ɺϳ���ϩ��2CO2 (g) +6H2 (g) ![]() C2H4(g)+4H2O(g)��0.1 MPaʱ����n(CO2)��n(H2)=l��3Ͷ�ϣ���ò�ͬ�¶���ƽ��ʱ��ϵ�и�����Ũ�ȵĹ�ϵ��ͼ��������������ȷ����
C2H4(g)+4H2O(g)��0.1 MPaʱ����n(CO2)��n(H2)=l��3Ͷ�ϣ���ò�ͬ�¶���ƽ��ʱ��ϵ�и�����Ũ�ȵĹ�ϵ��ͼ��������������ȷ����

A. �÷�Ӧ�ġ�H<0
B. ����b����H2O
C. N���M������״̬��c(H2)��һ��
D. �����������䣬T1�桢0.2 MPa�·�Ӧ��ƽ��ʱc(H2)��M���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����CaSO4����O2��ȼ�Ϸ�Ӧ��һ�ָ�Ч����ࡢ���õ�����ȼ�ռ�������ͼ1��ʾ��
ȼ�����з�Ӧ�� 1/4CaSO4(s�� + H2(g�� = 1/4CaS(s�� + H2O(g�� ��H1 (����Ӧ��
��Ӧ�� CaSO4(s�� + H2(g�� = CaO(s�� + SO2��g��+ H2O(g�� ��H2 (����Ӧ��
�������з�Ӧ��1/2 CaS(s�� + O2(g�� = 1/2CaSO4(s�� ��H3

��1��������Ӧ���з�����Ӧ�Ļ�ѧ����ʽ��_____________________��
��2��ȼ������SO2���ʵ����������¶�T��ѹǿp (MPa���ı仯����ͼ2����ͼ2�п��Եó�������Ҫ���ɣ�
�������������䣬�¶�Խ�ߣ�SO2����Խ�ߣ�
��________________________________________________________��
��_________________________________________________________��
��ͼ2��Ϊ����SO2���ŷ������ɲ�ȡ�Ĵ�ʩ��______________________��
��3����ȼ�ռ����п�ѭ�������ʳ�CaSO4��CaS�⣬����_________��д���ƣ���
��4����һ�������£�CO����ױ���Ӧ�����䱽����λ������һ��ȩ��������Ľṹ��ʽΪ___________��
��5���������Ȼ��٣�PdCl2����Һ��ȥH2�е�CO���������ʵ��װ��ͼ��
��ע��CO + PdCl2 + H2O �� CO2+ Pd + 2HCl��______________________

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʯī�缫������е��ʵ��
ʵ��һ | ʵ��� | |
װ�� |
|
|
���� | a��d����ֽ����;b�����,�ֲ���ɫ;c�������Ա仯 | ����ʯī�缫���������ݲ���;n�������ݲ������� |
���ж�ʵ������Ľ��ͻ��Ʋⲻ�������ǣ� ��
A. a��d����2H2O+2e-=H2��+2OH- B. b����2Cl--2e-=Cl2��
C. c�������˷�Ӧ��Fe-2e-=Fe2+ D. ����ʵ��һ��ԭ��,ʵ�����m��������ͭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾCalanolide A��һ�ֿ�HIVҩ����й���Calanolide A��˵����ȷ����

A.��������3������̼ԭ��
B.����������̼ԭ��һ����ͬһƽ����
C.�����ʿɷ���ȡ�����ӳɡ���ȥ��Ӧ
D.1 mol��������NaOH��Һ��ַ�Ӧ�������3 mol NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ԫ����(Ce)��һ����Ҫ��ϡ��Ԫ�ء�
(1) ��ҵ���÷�̼���(��Ҫ�ɷ�ΪCeFCO3)�Ʊ�CeO2�����չ����з�������Ҫ��Ӧ�Ļ�ѧ����ʽΪ________��

(2) ��֪CeCl3��7H2O�ڿ������ױ��������ļ��棬��Ӧ���£�
�� CeCl3��7H2O(s)=CeCl3(s)��7H2O(g)����H1��a kJ��mol��1
�� 4CeCl3(s)��O2(g)��14H2O(g)=4Ce(OH)4(s)��12HCl(g)����H2��b kJ��mol��1
�� Ce(OH)4(s)=CeO2(s)��2H2O(g)����H3��c kJ��mol��1
��4CeCl3��7H2O(s)��O2(g)=4CeO2(s)��12HCl(g)��22H2O(g)����H��________��
(3) CeO2������β����������������Ҫ������������ԭ����ͼ1��ʾ��д������1������Ӧ�Ļ�ѧ����ʽ��________________________________��

(4) ��֪Ce(OH)4���ֽ⣬����ͼ2��ʾװ�õ��CeCl3��Һ���������Ƶ�CeO2���������з�Ӧ��ϵ��pH��ʱ��t�ı仯������ͼ3��ʾ��
�ٵ�����1h����Һ��pHѸ���½�������ԭ����________��
�����������CeO2��ԭ����________��
(5) �����£�����Һ��ij����Ũ����1.0��10��5ʱ������Ϊ�����ӳ�����ȫ����Na2C2O4��Һ�Ե���ķ�Һ�����õ�Ce2(C2O4)3���壬��Ӧ������Һ��c(C2O42��)ԼΪ________��(��֪25 ��ʱ��Ksp[Ce2(C2O4)3]��1.0��10��25)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ���ش����⡣
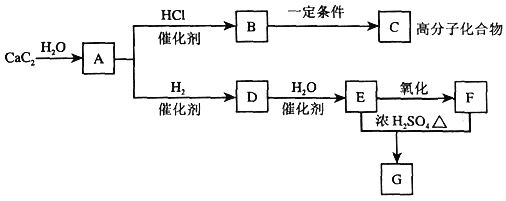
��1��B�����������ŵ������ǣ�_______��
��2����ӦB��C�Ļ�ѧ����ʽ��________����ӦD��E�Ļ�ѧ����ʽ��_______��
��3��B��C�ķ�Ӧ��������______��
��4��д��ʵ������ȡA�Ļ�ѧ����ʽ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ������˵����ȷ����
A.0.5 mol �ۻ�(As4S4)���ṹ��ͼ������NA��S��S�� 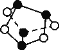
B.17 g��(��14CH3)������������Ϊ8NA
C.��״���£�33.6 L���ȼ����к�����ԭ�ӵ���ĿΪ3NA
D.�����£�16.8 g Fe������ˮ������ȫ��Ӧ��ת�Ƶĵ�����Ϊ0.6NA
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com