
| A���������ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ���壬��������һ����CO32�� |
| B������KSCN��Һ�������������ٵμӼ���������ˮ����Һ��ΪѪ��ɫ����������һ����Fe2+ |
| C������BaCl2��Һ��������ɫ�������ټ��������ϡ�����ữ���������ܽ⣬��������һ����SO42�� |
| D���ھƾ��ƻ��������գ�����Ϊ��ɫ����������һ������Ԫ�أ�һ��������Ԫ�� |
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| KMnO4������ҺŨ�� ��mol��L-1�� | ��Һ��ɫ����ʱ�䣨min�� | | ||
| ��һ�� | �ڶ��� | ������ | ||
| 0.02 | 14 | 13 | 11 | |
| 0.002 | 6.7 | 6.6 | 6.7 | |
| KMnO4������Һ | H2C2O4��Һ | ||
| Ũ��/ mol/L | ���(ml) | Ũ��/ mol/L | �����ml�� |
| 0.02 | 2 | b | 4 |
| a | 2 | c | 4 |
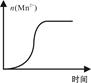
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������Һ�м���KI��Һ��ԭ�е������������ʵ������� |
| B�������Һ�еμ�ϡ����������Һ���������ְ�ɫ���� |
| C������Һ��c(Cl��)��0.6 mol��L��1�������Һ��pHΪ1 |
| D�������Һ�м���������ۣ�ֻ�����û���Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ������ | H����K����Al3����NH4+��Mg2�� |
| ������ | Cl����Br����OH����CO32-��AlO2- |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
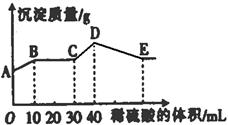
| A��AB�η�����Ӧ�����ӷ���ʽΪ��Ba2++SO42-=BaSO4�� |
| B��E���Ӧ������ϡ��������Ϊ70 ml |
| C��D���ʾ�ij����Ļ�ѧʽΪAl(OH)3��BaSO4 |
| D��E�������A�����������2.33g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
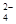 ����ѡ��Ba(OH)2��HCl��K2CO3�����Լ��������²��������
����ѡ��Ba(OH)2��HCl��K2CO3�����Լ��������²�������� 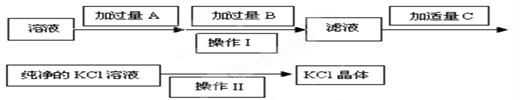
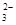 ��OH����HCO
��OH����HCO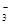 ��Cl���������е������֡�Ϊ��ȷ����Һ����ɣ����������²�����
��Cl���������е������֡�Ϊ��ȷ����Һ����ɣ����������²������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��HCO3�� | B��SO42�� | C��SO32�� | D��OH- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com