
���� ��1��������������백ˮ�����ȣ��õ������Ȼ����Һ��笠�����ˮ�⣬��Һ��ʾ���ԣ�
��2�����ݵ���غ��Լ��Ȼ����ʾ���Եĵ������ش�
��3���ڵμ�����Ĺ����У���Ϊ�����������������������Ͱ�ˮǡ����ȫ��Ӧ��������ݴ˻ش�
��4����Һ���������ȵ�ϡ����Ͱ�ˮ��϶��ɣ���ǡ�ó����ԣ�˵����Һ���Ȼ�狀Ͱ�ˮ�Ļ����ݴ˻ش�
��� �⣺��1��������������백ˮ�����ȣ��õ������Ȼ����Һ��笠�����ˮ�⣬��Һ��ʾ���ԣ�����Ũ�ȴ�С��ϵ�ǣ�c��Cl-����c��NH4+����c��H+����c��OH-�����ʴ�Ϊ���c��Cl-����c��NH4+����c��H+����c��OH-����
��2����Һ�����ԣ���c��Cl-��+c��OH-��=c��NH4+��+c��H+���ɵ�c��Cl-��=c��NH4+������Һ�����ԣ�˵����Һ���Ȼ�狀Ͱ�ˮ�Ļ�������ˮʣ�࣬�ʴ�Ϊ�����ڣ�С�ڣ�
��3���ڵμ�����Ĺ����У���Ϊ�����������������������Ͱ�ˮǡ����ȫ��Ӧ����������Dz���ʲô����£�һ�����ڵ���غ㣺c��OH-��+c��Cl-��=c��NH4+��+c��H+������ѡB��
��4����ˮΪ������ʣ�������Һ���������ȵ�ϡ����Ͱ�ˮ��϶��ɣ���ˮŨ�ȴ�������Ũ�ȣ���С�ڻ���ڣ�����Һ�����ԣ������ڻ��ǰ����c ��H+�����ڼ���c��OH-�����кͺ����ʾ���ԣ�
�ʴ�Ϊ��С�ڣ����ڣ�
���� �����ۺϿ��������ˮ�⡢������ʵĵ����Լ�����Ũ�ȵĴ�С�Ƚϣ���Ŀ�ѶȽϴ�ע����������ˮ���Լ�������ʵ�������������ձȽ�����Ũ�ȴ�С˳��ķ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2A+3B?2C | B�� | 2A+3B?3C | C�� | 3A+2B?3C | D�� | 3A+2B?2C |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  CO2���� | B�� | 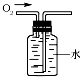 �ռ�O2 | C�� | 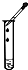 �μ�Һ�� | D�� | 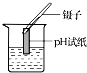 ����ҺpH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��ѧʽ | AgCl | AgBr | AgI | Ag2S | Ag2CrO4 |
| ��ɫ | ��ɫ | dz��ɫ | ��ɫ | ��ɫ | ��ɫ |
| Ksp | 1.8��10-10 | 5.0��10-13 | 8.3��10-17 | 2.0��10-48 | 1.8��10-10 |
| A�� | KBr | B�� | KI | C�� | K2S | D�� | K2CrO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ͼ�Ǽ���ȼ�ϵ�صĽṹʾ��ͼ�������ڴ����������ṩ���ӣ�H+���͵��ӣ����Ӿ����·�����Ӿ��ڵ�·������һ����������Ӧ������ܷ�ӦΪ CH4+2O2��CO2+2H2O������˵������ȷ���ǣ�������
ͼ�Ǽ���ȼ�ϵ�صĽṹʾ��ͼ�������ڴ����������ṩ���ӣ�H+���͵��ӣ����Ӿ����·�����Ӿ��ڵ�·������һ����������Ӧ������ܷ�ӦΪ CH4+2O2��CO2+2H2O������˵������ȷ���ǣ�������| A�� | ��缫Ϊ��صĸ�����a��ͨ��������Ǽ��� | |
| B�� | ��ع���ʱ���·���ӴӸ��������������ڵ�·���Ӵ��������� | |
| C�� | ������ӦʽΪ��CH4+2H2O-8e-��CO2+8H+ | |
| D�� | ������ӦʽΪ��O2+4H++4e-��2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| ѡ�� | A | B | C | D |
| ���� | �ӵ�ʳ�� | ʯӢɰ | ��84������Һ | ˮ���� |
| ��Ҫ�ɷ� | KIO3 | SiO2 | Ca��ClO��2 | NaSiO3 |
| ��; | Ԥ����ȱ���� | ������� | �������� | ľ�ķ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͬ | B�� | H2SO4��Һ���ĵ�NaOH�� | ||
| C�� | HCl��Һ���ĵ�NaOH�� | D�� | ���Ƚ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com