
����Ŀ��ʵ����������̼���ƾ���(Na2CO3��10H2O)������0.5mol�qL-1��̼������Һ1000mL���ش���������
(1)��Ҫ����̼���ƾ��������Ϊ___________g����ѡ������ƿ�Ĺ��Ϊ___________ mL��
(2)ϴ�Ӳ����У���ϴ���ձ������ҺҲע������ƿ����Ŀ����__________________________________��
(3)���ݵ���ȷ�����Ǽ���������ˮ����̶�����1~2cmʱ������_________��ˮ��Һ����̶������С�
(4)�ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ������ķ�����__________(����ĸ)��
a.��������
b.��������Һ�壬ʹ��Һ����̶�������
c.���������һ������̼���ƾ���
d.С�ļ�������ƿ����������ʹ��Һ����̶�������
(5)����ʱ���в����ᵼ��������ҺŨ��ƫ�ߵ���_____________(����ĸ)��
a.ת����ʱ��������Һ����
b.����ʱ���Ӷ�ȡ�̶�
c.����ƿ������ˮϴ����δ����
d.����ʱҺ�泬���˿̶���
(6)���������Ƶ���Һ��ȡ��10mL�����к�Na2CO3��������_____________g��
���𰸡�143.0 1000 ʹ������ȫת�Ƶ�����ƿ�� ��ͷ�ι� a b 0.53
��������
������Һ���ƵIJ�������������⣻���c=![]() ������
������
(1) ������0.5mol�qL-1��̼������Һ1000mL����Na2CO3�����ʵ���n=cV=1L��0.5molL-1=0.5mol��Na2CO310H2O�����ʵ�������Na2CO3�����ʵ���������Na2CO310H2O������0.5mol��286g/mol=143.0g����ѡ������ƿ�Ĺ��Ϊ1000mL��
(2) ����c=![]() ��֪��Ҫʹ������ҺŨ��ȷ������Ҫ����ȫ��ת�Ƶ�����ƿ�У�����ϴ�Ӳ����У���ϴ���ձ������ҺҲע������ƿ����Ŀ����ʹ������ȫת�Ƶ�����ƿ�У�
��֪��Ҫʹ������ҺŨ��ȷ������Ҫ����ȫ��ת�Ƶ�����ƿ�У�����ϴ�Ӳ����У���ϴ���ձ������ҺҲע������ƿ����Ŀ����ʹ������ȫת�Ƶ�����ƿ�У�
(3) ���ݵ���ȷ�����Ǽ���������ˮ����̶�����1-2cmʱ�����ý�ͷ�ιܼ�ˮ��Һ����̶������У�
(4) �ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ�����ʵ��ʧ�ܣ������ȣ���Ҫ��������,�ʴ�Ϊa��
(5) a��ת��ʱ��������Һ���������²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���a����
b������ʱ���Ӷ�ȡ�̶ȣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���b��ȷ��
c������ƿ������ˮϴ����δ��������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬��c����
d������ʱҺ�泬���˿̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���d����
�ʴ�Ϊb��
(6) ��Һ���о�һ�ԣ�Ũ��������أ����Դ��������Ƶ���Һ��ȡ��10mL������Na2CO3�����ʵ���Ũ����0.5mol/L����̼���Ƶ�����Ϊ��0.01L��0.5mol/L��106g/mol=0.53g��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��������������ȷ����
A.����ͼ���ж϶�����![]() �������£���ѧ��Ӧƽ�ⳣ������
�������£���ѧ��Ӧƽ�ⳣ������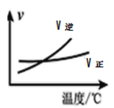
B.ͼ��ʾѹǿ�Կ��淴Ӧ![]() ��Ӱ����
��Ӱ����![]()
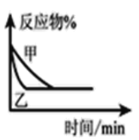
C.ͼ�ɱ�ʾ������Һ��ͨ�백����������������Һ�����Եı仯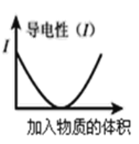
D.����ͼ������ȥ![]() ��Һ�е�
��Һ�е�![]() �ɲ�������Һ�м�������
�ɲ�������Һ�м�������![]() ����Һ��pH��4���Ҽ���
����Һ��pH��4���Ҽ���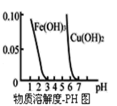
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NH3���й㷺����;��ʵ���ҳ�����ͼ��ʾװ����ȡ���ռ�NH3��

��1��ʵ������NH4Cl��Ca(OH)2��ȡNH3�Ļ�ѧ����ʽΪ______��
��2������NH3Ӧѡ�õĸ������______��
��3��ͼ1�з������ռ�NH3��װ�ÿ�ѡ��ͼ2�е�______������ţ���
��4��β������װ����ʹ�õ���©����������______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��D��E��F����Ҫ���л�����ԭ�ϡ���A�IJ�����һ������ʯ�ͻ�����չˮƽ�ı�־��A����Է�������Ϊ28��B������ȼ�Ϻ��ܼ���FΪ����ζ����״Һ�塣����֮���ת����ϵ����ͼ��

��1��A�Ľṹ��ʽ��______����Ӧ������______���Ӧ���ͣ���
��2��B��������______��
��3����Ӧ�ڵĻ�ѧ����ʽ��______��
��4����Ӧ�ܵĻ�ѧ����ʽ��______��
��5������˵������ȷ����______������ţ���
a��A��B�����ܱ����Ը��������Һ����
b���ñ���Na2CO3��Һ�ܳ�ȥF�л��е�����B��E
c�������п�����E��ȥˮ���е�ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о����������������ڴ����к������ӵ������ʱ���漰���·�Ӧ��
2NO2(g)��NaCl(s)![]() NaNO3(s)��ClNO(g)��K1����H1<0(��)
NaNO3(s)��ClNO(g)��K1����H1<0(��)
2NO(g)��Cl2(g)![]() 2ClNO(g)�� K2�� ��H2<0 (��)
2ClNO(g)�� K2�� ��H2<0 (��)
(1)4NO2(g)��2NaCl(s)![]() 2NaNO3(s)��2NO(g)��Cl2(g)��ƽ�ⳣ��K��________(��K1��K2��ʾ)��
2NaNO3(s)��2NO(g)��Cl2(g)��ƽ�ⳣ��K��________(��K1��K2��ʾ)��
(2)Ϊ�о���ͬ�����Է�Ӧ(��)��Ӱ�죬�ں��������£���2 L�����ܱ������м���0.2 mol NO��0.1 mol Cl2��10 minʱ��Ӧ(��)�ﵽƽ�⡣���ƽ���n(Cl2)��2.5��10��2 mol��10 min����(ClNO)��________________����NO��ת������1��________�������������ֲ��䣬��Ӧ(��)�ں�ѹ�����½��У�ƽ��ʱNO��ת������2 ________��1(����>������<����������)��
(3)�����ǹ�ҵ���������Ҫԭ��֮һ�������������з�������Ҫ��Ӧ���£�
I. 4NH3(g) + 5O2(g)![]() 4NO(g) + 6H2O(g) ��H =-906 kJ��mol��1
4NO(g) + 6H2O(g) ��H =-906 kJ��mol��1
II.4NH3(g) + 3O2(g) 2N2(g) + 6H2O(g) ��H = -1266 kJ��mol��1
��1L���ܱ������г���1 mol NH3��2 mol O2������й����ʵ����ʵ����뷴Ӧ�¶ȵĹ�ϵ��ͼ��ʾ��

�ٴ����������У������˵��¶�Ϊ_________________(����T1������T2�� ����T����)
��д��N2��O2����NO���Ȼ�ѧ����ʽ________��
���¶�ΪT2ʱ����ӦII��ƽ�ⳣ��K =_________________(ֻ����ʽ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���![]() mol
mol![]() ��������ˮ���100mL��Һ������Һ�м����������ʣ��йؽ�����ȷ����
��������ˮ���100mL��Һ������Һ�м����������ʣ��йؽ�����ȷ����
A.����50mL1![]() ����Ӧ������
����Ӧ������![]()
B.����![]() molCaO����Һ��
molCaO����Һ��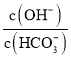 ��С
��С
C.����50mL![]() ����ˮ�������
����ˮ�������![]() ����
����
D.����![]() mol
mol![]() ���壬��Ӧ��ȫ����Һ������
���壬��Ӧ��ȫ����Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ���Ũ��Ϊ0.1 mol��L��1��Na2CO3��Һ100 mLʱ�����в�����ȷ����(����)
A. ��������ƽ��ȡ1.06 g��ˮ̼���ƣ����ձ��м�����ˮ�ܽ⣬����ȴ�����º���Һת�Ƶ�����ƿ��
B. ��ʹ������ƿǰ����������ƿ�Ƿ�����Լ�ƿ�����Ƿ�©ˮ
C. ����ʱ������ˮ�����̶��ߣ����ý�ͷ�ι�С���������ಿ��
D. ����ʱ�������ӣ���������Һ��Ũ�Ƚ�ƫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��CuSO4��Һ�м���һ������Na2SO3��NaCl��Һ���ȣ�����CuCl������д������CuCl�����ӷ���ʽ________________________________
(2)������H2CrO4��N2H4��ԭΪCr3����ͬʱ�ų�����Ⱦ�����壬д��������Ӧ�����ӷ���ʽ_________�����������뻹ԭ��������ʵ���֮��Ϊ________��
(3)��ȥ��Һ�е�AsCl3�����ô�������(NaH2PO2)��ԭAsCl3����������ɫ�����������H3PO3���÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ______________________�������ͻ�ԭ�������ʵ���֮��Ϊ________������������________��
(4)FeS��ˮ��Һ��Cl2���������������Һ�еμ�BaCl2�в��ܽ�������İ�ɫ�������ɣ���ˮ��Һ��FeS��Cl2��Ӧ�����ӷ���ʽΪ_______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ A2+3B22C �ķ�Ӧ�����»�ѧ��Ӧ���ʵı�ʾ�У���Ӧ����������
A.��(A2) = 0.4 mol��L-1��s-1B.��(B2) = 0.8 mol��L-1��s-1
C.��(C) = 0.6 mol��L-1��s-1D.��(B2) = 4.2 mol��L-1��min-1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com