
����Ŀ������̿�������ǹ�ҵ������Ҫ����֮һ�����������£�
![]()
����������⣺
��1�����������£�NaNO2��Һֻ�ܽ� I������ΪI2��ͬʱ����NO��д����Ӧ�ٵ����ӷ���ʽ���������ת�Ƶ���Ŀ�ͷ���______________��
��2��������I2�⾭��������ת��ΪI����IO3����ת��ΪI2�Ĺ��̣���������Ŀ����_______��
��3����Ӧ�ڷ���ʱ�����ڵ���ˮ���ܽ�Ȳ�����Һ�ײ��й������ɣ����ڷ�Ӧ���ȣ���ʱ��Һ�Ϸ�����_____������ɫ�������塣��ˣ���Ӧ����Ҫ��______�����½��С�
��4��ʵ���Ҵӷ�Ӧ��������Һ��ȡ�⣬�ɼ���CCl4_______����������ƣ��⣬���ѵ��ˮ��Һ����ȡ����������________�����������ƣ�����������Һ��
��5������̿��������I2Ҳ������NaHSO3����ת��ΪI�����÷�Ӧ����������Ϊ_______���������ţ���
��֪NaHSO3��Һ�������ԣ��Դ�ƽ��ĽǶȽ���ԭ��____________��
��0.1mol/L��NaHSO3��Һ�м��백ˮ�����ԣ����жϣ�c(Na+)____c(SO32�C)+ c(HSO3�C)+ c(H2SO3)������>������<������=������
���𰸡�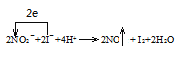 ������Ũ�ȣ������� ��ɫ ��ˮԡ���ߵ��� ��ȡ ��Һ©�� SO42�� NaHSO3��Һ��HSO3�C����ˮ���Լ��ԣ����ܵ����H+ �����ԣ����������̶ȴ���ˮ��̶ȣ�����NaHSO3��Һ�������� =
������Ũ�ȣ������� ��ɫ ��ˮԡ���ߵ��� ��ȡ ��Һ©�� SO42�� NaHSO3��Һ��HSO3�C����ˮ���Լ��ԣ����ܵ����H+ �����ԣ����������̶ȴ���ˮ��̶ȣ�����NaHSO3��Һ�������� =
��������
(1)�������ƾ��������ԣ������Ӿ��л�ԭ�ԣ����������£����߷���������ԭ��Ӧ����һ�������͵��ˮ���ݴ˷������
(2)�����е�Ԫ�ؾ�����I2��I-��IO3-��I2�Ĺ��̿���������Ũ�ȣ�
(3)����ˮ�е��ܽ�Ȳ�������������
(4)����ˮ�е��ܽ�Ȳ����������л��ܼ���
(5)I2������NaHSO3����������ԭ��Ӧ��NaHSO3�������������ƣ�NaHSO3��Һ��HSO3�C����ˮ�����ܵ��룻���������غ�������ݴ˷������
(1)�������ƾ��������ԣ������Ӿ��л�ԭ�ԣ����������£����߷���������ԭ��Ӧ����һ�������͵��ˮ�����ӷ���ʽΪ��2NO2-+4H++2I-�T2NO��+I2+2H2O������ת�Ƶ���Ŀ�ͷ�����Ա�ʾΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(2)�����У���Ԫ�ؾ�����I2��I-��IO3-��I2�ı仯���̣�����������ԭ���ǿ���������Ũ�ȣ��ﵽ������Ԫ�ص�Ŀ�ģ��ʴ�Ϊ��������Ԫ�أ�
(3)��Ӧ�ڷ���ʱ����Һ�ײ����Ϻ�ɫ�Ĺ������ɣ���������������ʱ��Һ�Ϸ�������ɫ�ĵ����������ԣ���Ӧ����Ҫ�ڱ�ˮԡ���ߵ��������½��У��ʴ�Ϊ����ɫ (4). ��ˮԡ���ߵ��£�
(4)ʵ���Ҵӷ�Ӧ��������Һ��ȡ�⣬����ˮ�е��ܽ�Ȳ����������л��ܼ����ɼ���CCl4��ȡ�⣬���ѵ��ˮ��Һ����ȡ��������ȡ���÷�Һ©������������Һ���ʴ�Ϊ����ȡ����Һ©����
(5)����̿��������I2Ҳ������NaHSO3����ת��ΪI������ΪNaHSO3�ܹ������������������ƣ���˸÷�Ӧ����������ΪSO42����NaHSO3��Һ��HSO3�C����ˮ�����ܵ��룬ˮ��ʹ���Լ��ԣ�����ʹ�������ԣ����������̶ȴ���ˮ��̶ȣ�����NaHSO3��Һ�������ԣ���0.1mol/L��NaHSO3��Һ�м��백ˮ�����ԣ���Һ��c(H+)=c(OH-)�����������غ���c(Na+)=c(SO32-)+c(HSO3-)+c(H2SO3)���ʴ�Ϊ��SO42����NaHSO3��Һ��HSO3�C����ˮ���Լ��ԣ����ܵ����H+ �����ԣ����������̶ȴ���ˮ��̶ȣ�����NaHSO3��Һ�������ԣ�=��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Dzⶨij��Ѫ����FeSO47H2O������Ԫ�غ���������ͼ�������������������գ�

(1)�������Ҫ100mL1mol/L��ϡ���ᣬ��98.3%����=1.84g/cm3��Ũ�������ƣ����õIJ�����������Ͳ���ձ�����ͷ�ιܡ�_________��__________��H2O2��������_____________��
(2)�����һϵ�в��������ǣ��ٹ��ˢ�ϴ�Ӣ�_________����ȴ�ݳ������ز�����������Ŀ����____________________��֤���������Ƿ�ϴ�Ӹɾ��IJ���________________��
(3)����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����________g(�ú�a�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�����û����зϴ���(��CoO��Co��Al2O3������FeO)��ȡ������CoSO4�ֲ�Ʒ�Ĺ����������£�

��֪��(i)��ؽ��������γ��������������pH��Χ���±���ʾ��

(��)Al(OH)3�ڼ��������¿�ʼ�ܽ�ʱ��pHΪ7.8����ȫ�ܽ�ʱ��pHΪ11��
�ش��������⣺
(1)д��H2O2�ĵ���ʽ��___________��
(2)���д�ʩһ������߲���I��A13+��Co2+�Ľ�ȡ�ʵ���___________(����)
a�����ϴ�����ĥΪϸ����
b������I�е��������98%��Ũ����
c���ʵ���߽�ȡʱ���¶�
(3)������У�д����������������Fe2+�����������ӷ���ʽ��___________����������������������pH=3������ɵĺ����___________��
(4)������м�K2CO3Ӧ����pH�ķ�ΧΪ___________��
(5)�ⶨCoSO4�ֲ�Ʒ���ܵ����������IJ������£�ȷ��ȡag��Ʒ���Ⱦ�Ԥ������Ȼ���������ı����ᣬ�ڼ�������£������μ�KNO2��Һֱ�����������ɲ����������K3[Co(NO2)6]���پ����ˡ�ϴ�Ӽ������������������Ϊbg��
��KNO2��Һ����������Co2+�����ӷ���ʽΪ___________(��֪KNO2����ԭΪNO)��
�ڴֲ�Ʒ����Ԫ�ص���������Ϊ___________(Mr{K3[Co(NO2)6]}=452���г�����ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����Ʊ����ᶡ����װ����ͼ��ʾ�����з����������

A. ����������������߶�����ת����
B. �ᴿ���ᶡ����Ҫ����ˮ������������Һϴ��
C. ������a����������������
D. �����ᶡ���ķ�Ӧ�¶ȳ���100�治����ˮԡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڱ�״���£�0.5molHClռ�е������_______��33.6L H2�����ʵ�����_______��16g O2�������_______��44.8LN2�к���N2��������________��34g������__________gˮ������ͬ��Ŀ����ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������У�����Cԭ�ӿ϶���ͬһƽ�������
A. CH3CH��CHCH2CH3 B. CH3CH��CHCH2C��CH
C. ![]() D.
D. ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(Se)��ͭ(Cu)�������������й㷺��Ӧ�á������������������ϡ��������ҵ�Ĵ�����Ҳ�Ƕ���������Ӫ��Ԫ�غͶ�ֲ�������Ӫ��Ԫ�صȡ��Ȼ���ͭ(CuCl)�㷺Ӧ���ڻ�����ӡȾ����Ƶ���ҵ��CuCl�����ڴ���ˮ��������������Ũ�Ƚϴ����ϵ���ڳ�ʪ��������ˮ���������Ժ���ͭ(��Ҫ�ɷ���Cu������CuO)Ϊԭ�ϣ���������������ֽ⼼������CuCl�Ĺ��չ���������ʾ��

��ش��������⣺
(1)��������еõ�����������ֻ��һ�֣������Ļ�ѧʽ��____________��
(2)д�����������Ҫ��Ӧ�����ӷ���ʽ��____________________________________��
(3)����ݰ�����pH��2����Һ��ϴ��ˮϴ������������ϴ���õ�����__________(д�������)��
(4)���������У�����͢ߵ�������_____________��
(5)SeΪ��A��Ԫ�أ����Ҷ���������ͭ������ˮ��Һ������������(Na2SeSO3)��Һ��Ӧ�ɻ����������ͭ�����������ƻ�������Se�ľ��ƣ�д������������(Na2SeSO3)��H2SO4��Һ��Ӧ�õ������Ļ�ѧ����ʽ��_____��
(6)�Ȼ���ͭ�������¶ȡ���ҺpH��ϵ����ͼ��ʾ����ͼ���������̻������Ȼ���ͭ�Ĺ����У��¶ȹ���Ӱ��CuCl���ʵ�ԭ����____________________________________���¶ȹ��ߡ�pH����Ҳ��Ӱ��CuCl���ʵ�ԭ����_______________________________��

(7)��NaHS����ˮ�����ij����������Դ�����ҵ��ˮ�е�Cu2������֪��25��ʱ��H2S�ĵ���ƽ�ⳣ��Ka1��1.0��10��7��Ka2��7.0��10��15��CuS���ܶȻ�ΪKsp(CuS)��6.3��10��36����ӦCu2��(aq)��HS��(aq) ![]() CuS(s)��H��(aq)��ƽ�ⳣ��K��__________(�������1λС��)��
CuS(s)��H��(aq)��ƽ�ⳣ��K��__________(�������1λС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������Ҫ�Ĺ���Ԫ�ء�
(1)��λ��Ԫ�����ڱ��������ڢ��壬���̬ԭ����δ�ɶԵ��Ӹ���Ϊ___________��
(2)��̬Fe3+�ĺ�������Ų�ʽ___________
(3)��������һ�ִ��Բ��ϣ���ҵ���Ʊ�ʱ������ˮ�ⷨ���Ʊ�ʱ����������(CO(NH2)2)�������Ƶȼ������ʡ����ط����������ǽ���Ԫ�صĵ縺���ɴ�С��˳����___________����������������������Ŀ֮��Ϊ___________����������̼ԭ�ӵ��ӻ�����___________��
(4)������Ҳ��ʹ�ó��������Ʊ�ʱ�����백(NH3)������(N2H4)�������֪��(NH3�۵㣺��77.8%�桢�е㣺��33.5%��)������(N2H4�۵㣺2�桢�е㣺113.5��C)�������۷е�ߵ͵���Ҫԭ��______________________��
(5)Co(NH3)5BrSO4���γ������ܵ�������֪Co3+����λ��Ϊ6��Ϊȷ����������Ľṹ���ֶ�����������������ʵ�飺�ڵ�һ���������Һ�м�����������Һ������ɫ���������һ������������Ϊ___________���ڵڶ����������Һ�м�����������Һ��������ɫ��������ڶ�������������Ϊ___________��

(6)��������̼�ܽ���r-Fe���γɵ�һ�ּ�϶�����壬���ԣ��侧��Ϊ���������ṹ������ͼ��ʾ��������ʵĻ�ѧʽΪ___________����Ʒ���ܶ�Ϊdg��cm��3���������������̼ԭ�ӵľ���Ϊ___________pm(�����ӵ�������ֵ��NA��ʾ��д����ļ���ʽ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������(K2FeO4)��һ�ָ�Ч��ˮ��.��֪:K2FeO4 ������ˮ,����Һ����ɫ������ŨKOH ��Һ,��0����5����ǿ������Һ�н��ȶ�.ijС��ͬѧ����ͼװ���Ʊ���̽��
K2FeO4 ������.�Ʊ�ԭ��:
3Cl2��2Fe(OH)3��10KOH��2K2FeO4��6KCl��8H2O,װ����ͼ��ʾ(�г�װ����)

(1)ʢ�Ŷ������̵���������___________________,װ��C��������____________________��
(2)װ��A �з�Ӧ�Ļ�ѧ����ʽ��________________________________________��
(3)ʵ��ʱ���ñ�ˮԡ��ԭ����____________________,��װ�ô���һ������ȱ��,��ָ��____________��
(4)K2FeO4 �ֲ�Ʒ����Fe(OH)3��KCl������,һ����75���Ҵ�����ϴ��,��Ŀ����_____________________��
(5)��������֪,K2FeO4 �ܽ� Mn2�������� MnO4��.��С���������ʵ�������֤:

�ر�K,���ձ���Һ���ɫ,���ձ���Һ����ɫ.��������ձ���Һ�ʻ�ɫ��ԭ��,��Ҫ���Լ���__________��д��K2FeO4 ����Mn2�� �����ӷ���ʽ: ___________________.
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com