
����Ŀ��ʳƷ����Ĥ�����ʷֱ�Ϊ����ϩ(PE)��������ϩ(PVC)����ƫ������ϩ(PVDC)�����ࡣ����PE��PVDC�ǰ�ȫ�ġ�PVC�������DZ��Σ����Ҫ��Դ���������棺��Ʒ������ϩ������������ӹ�������ʹ�õļӹ����������༰������
(1)д������ϩ������ϩ�ֱ���������ϩ�;�����ϩ�Ļ�ѧ����ʽ��
��ϩ������ϩ(PE)��__________________________________________
����ϩ��������ϩ(PVC)��____________________________________
�ճ��õ�ʳƷ����Ĥ��ѡ��____________��
(2)Ŀǰ���ֵ�����ճ��������ճ�͵������̻������ͳ��ߣ������ܾ��DZ�����һ�ֽ������ظ�������Ϳ�㣬ʵ���Ͼ��DZ���Ϊ�����������ľ��ķ���ϩ�����ĵ����ķ���ϩ�Ľṹ��ʽ��_______________���ϳ��ظ����ķ�Ӧ������___________����Ӧ����ʽΪ_________________��
(3)���ķ���ϩ�ķ���Դ��һ��ʵ�����⡣1938�꣬��ѧ����³�������������о��ķ���ϩ�ľۺϷ�Ӧ�����ǽ��ķ���ϩ������������еļ�ѹ��ƿ�У�������ƿ�뷴Ӧ���ܵ�����Ӧ��(�ܵ�����Ӧ����϶�п���)��ͨ�������ϣ��ķ���ϩ���岢û��ͨ����Ӧ���У�������֤����ƿ������һ��û���٣�����Ҳû���ķ���ϩ�����ݳ�������ж���ź�ȴ�Ӹ�ƿ�������ķ���ϩ���صİ�ɫ��ĩ�����ķ���ϩ���ڸ�ƿ�з����˾ۺϷ�Ӧ����ȷ֤���ð�ɫ��ĩ���Ǿ��ķ���ϩ��1945�꣬�����Ű˾��ʼ�ڹ�ҵ��ģ���������ķ���ϩ���ɴ˿�����֪�÷�Ӧ��������___________��____________��____________������
(4)��ճ�����ڱ���һ������ķ���ϩͿ�㣬����ò�ճ���շ���ʱ����ճ��������˵����ȷ������______��
a.���ķ���ϩ������û��˫�� b.���ķ���ϩ�ĵ����Dz�������
c.���ķ���ϩ�Ļ�ѧ���ʺܲ����� d.���ķ���ϩ���۷е�ܵ�
���𰸡�![]()
![]() ����ϩ
����ϩ ![]() �Ӿ۷�Ӧ
�Ӿ۷�Ӧ ![]() ���� ��ѹ ���� ac
���� ��ѹ ���� ac
��������
(1)��ϩ������ϩ������̼̼˫�����ɷ����Ӿ۷�Ӧ���ɸ߾������ϩ������ϩ�ֱ���������ϩ�;�����ϩ�Ļ�ѧ����ʽ����ϩ������ϩ(PE)��![]() ������ϩ��������ϩ(PVC)��
������ϩ��������ϩ(PVC)��![]() ��������ϩ�������к����ճ��õ�ʳƷ����Ĥ��ѡ�þ���ϩ��
��������ϩ�������к����ճ��õ�ʳƷ����Ĥ��ѡ�þ���ϩ��
(2)�ķ���ϩ�Ľṹ��ʽ��CF2=CF2������̼̼˫�����ɷ����Ӿ۷�Ӧ���ɾۺ���ϳ��ظ����ķ�Ӧ�����ǼӾ۷�Ӧ����Ӧ����ʽΪ![]() ��
��
(3)���ķ���ϩ������������еļ�ѹ��ƿ�У��ܵ�����Ӧ����϶�п�������֪��Ӧ������Ϊ���¡���ѹ��������
(4)a.���ķ���ϩΪ�ķ���ϩ�ľۺ��������û��˫������a��ȷ��
b.���ķ���ϩ�ĵ����Dz����������������b����
c.���ķ���ϩ����̼̼˫��������FԪ�أ�Ϊ������������ʽ�Ϊ�ȶ�����ѧ���ʺܲ����ã���c��ȷ��
d.���ķ���ϩ���۷е�ϸߣ���d����
��ѡ��ac��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����ӦFe(s)��CO2(g) ![]() FeO(s)��CO(g)��ƽ�ⳣ��ΪK1����ӦFe(s)��H2O(g)
FeO(s)��CO(g)��ƽ�ⳣ��ΪK1����ӦFe(s)��H2O(g) ![]() FeO(s)��H2(g)��ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ�����
FeO(s)��H2(g)��ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ�����
700�� | 900�� | |
K1 | 1.47 | 2.15 |
K2 | 2.38 | 1.67 |
��ӦCO2(g)��H2(g) ![]() CO(g)��H2O(g)��ƽ�ⳣ��K����K��___(��K1��K2��ʾ)���������������֪����ӦCO2(g)��H2(g)
CO(g)��H2O(g)��ƽ�ⳣ��K����K��___(��K1��K2��ʾ)���������������֪����ӦCO2(g)��H2(g) ![]() CO(g)��H2O(g)��___��Ӧ(����ȡ����ȡ�)��
CO(g)��H2O(g)��___��Ӧ(����ȡ����ȡ�)��
��2��һ���¶��£���ij�ܱ������м����������۲�����һ������CO2���壬������ӦFe(s)��CO2(g) ![]() FeO(s)��CO(g) ��H��0��CO2��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
FeO(s)��CO(g) ��H��0��CO2��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
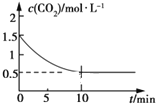
�ٸ������·�Ӧ��ƽ�ⳣ��Ϊ___��������������CO2����ʼŨ��Ϊ2.0 mol��L��1����ƽ��ʱCO2��Ũ��Ϊ___mol��L��1��
�����д�ʩ����ʹƽ��ʱ![]() �������___(�����)��
�������___(�����)��
A�������¶� B������ѹǿ
C���ٳ���һ������CO2 D���ټ���һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
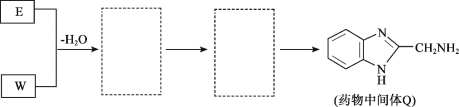
����Ŀ��ҩ���м���Q��ҽ�ò���PVA�ĺϳ�·����ͼ��

��֪��
��.��NH2+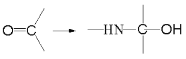
��.A�ķ���ʽ��C6H6
��.W���������������
IV.C��D��ȡ����Ӧ
��ش��������⣺
(1)A��B�ķ�Ӧ������___��
(2)B��C��������Ӧ���Լ�a��___��
(3)E�Ļ�ѧ������___��
(4)F�ĺ���̼̼˫����������ͬ���칹����___��(��˳���칹�壬����F)�����к˴Ź���������3�����շ壬���ܷ���������Ӧ�Ľṹ��ʽ��___��
(5)G��X�Ļ�ѧ����ʽ��___��
(6)W�ܷ����ۺϷ�Ӧ�����γɵĸ߷��ӻ�����Ľṹ��ʽ��___��
(7)������E+W��Q������ͼ��������(�����߿���д�����ʵĽṹ��ʽ)��___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ũ�����![]() ��
��![]() �����Ը����
�����Ը����![]() Ϊԭ�Ͻ���ʵ�飬װ������ͼ��ʾ��
Ϊԭ�Ͻ���ʵ�飬װ������ͼ��ʾ��
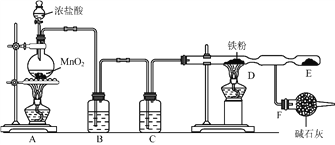
��װ��![]() ��ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ��_________________________________��
��ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ��_________________________________��
��װ��![]() ��
��![]() ��Ӧʢ�ŵ��Լ����Ʒֱ�Ϊ__________��_________����
��Ӧʢ�ŵ��Լ����Ʒֱ�Ϊ__________��_________����![]() װ�õ�����������������
װ�õ�����������������![]() ��
��![]() װ�ö�ֱ�ӽ���
װ�ö�ֱ�ӽ���![]() �ܣ�����ʵ������IJ��������___________________________��
�ܣ�����ʵ������IJ��������___________________________��
��ʵ��ʱ���ȵ�ȼ![]() ���ľƾ��ƣ�����������װ�ã��ٵ�ȼ
���ľƾ��ƣ�����������װ�ã��ٵ�ȼ![]() ���ƾ��ƣ�д��
���ƾ��ƣ�д��![]() �з�Ӧ�Ļ�ѧ����ʽ��_________________________________________��
�з�Ӧ�Ļ�ѧ����ʽ��_________________________________________��
��![]() װ�������������____________________��____________________��
װ�������������____________________��____________________��
�������۵�ʯ���������������Ƶ�Ư�۾���д����ҵ��Ư�۾���Ӧ�Ļ�ѧ����ʽ��
___________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��A��B��Cԭ���������ε��������ǵ�ԭ�ӵ�����������֮��Ϊ10��A��C�����ڱ���ͬ���壬Bԭ����������������Aԭ�Ӵ���������������������ȷ����(����)
A. ԭ�Ӱ뾶A��B��C
B. A���⻯����ȶ���С��C���⻯����ȶ���
C. C����������۵��A��������ĵ�
D. A��C���γ�ԭ�Ӿ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)HClO �����и�ԭ�Ӿ������ȶ��ṹ��д�� HClO �ĵ���ʽ_____��
(2)̼������ˮ��Һ�ʼ��ԣ��Է���ԭ��_____(�����ӷ���ʽ��ʾ)��
(3)ά���� B1 �Ľṹ��ʽ��ͼ��ʾ��ά���� B1 ��������ˮ�Ĺ��̣� ��Ҫ�˷��������������������»������_____��
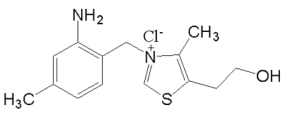
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����![]() ���Ȼ���XCl2 0.95g�����Һ������1mol/L����������Һ20mL���ܰ���������ȫ�����������Լ��㣺
���Ȼ���XCl2 0.95g�����Һ������1mol/L����������Һ20mL���ܰ���������ȫ�����������Լ��㣺
(1)X��������Ϊ_________��
(2)��X�ĺ���������Ϊ12����47.5gXCl2���������ӵ����ʵ�����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ⶨCu��NO3��2nH2O�Ľᾧˮ���������з����п϶������е��ǣ� ��
A.������Ʒ�����ȡ���ȴ������CuO
B.������Ʒ�����ȡ���ȴ������Cu��NO3��2
C.������Ʒ�����ȡ�����֪��������ˮ�Ȼ�������ˮ����������
D.������Ʒ����NaOH�����ˡ����ȡ���ȴ������CuO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����MgSO4��Al2(SO4)3�Ļ����Һ�У���μ���NaOH��Һ������ͼ���У�����ȷ��ʾ������Ӧ����(�������ʾ����NaOH��Һ��������������ʾ��Ӧ���ɳ���������) (����)
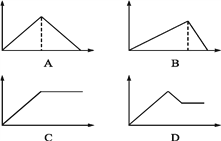
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com