
(��)�����£���ijһԪ��HA(�ס��ҡ�������������ͬ��һԪ��)��NaOH��Һ�������ϣ�������Һ�����ʵ���Ũ�Ⱥͻ����Һ��pH�����ʾ��
| ʵ�� ��� | HA�����ʵ��� Ũ��(mol��L��1) | NaOH�����ʵ��� Ũ��(mol��L��1) | ��Ϻ��� Һ��pH |
| �� | 0.1 | 0.1 | pH��a |
| �� | 0.12 | 0.1 | pH��7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 |
| pH��10 |
(1)�Ӽ����������������ж�HA��ǿ�ỹ�����
________________________________________________________________________��
(2)��������Һ��c(A��)��c(Na��)�Ĵ�С��ϵ��________��
A��ǰ�ߴ� B�����ߴ�
C��������� D�����ж�
(3)�ӱ���ʵ�����������û����Һ������Ũ���ɴ�С��˳����________________________________________________________________________��
(4)��������ʵ�����ݣ�д���û����Һ��������ʽ�ľ�ȷ���(��ʽ)��c(Na��)��c(A��)��________ mol��L��1��
(��)ij��Ԫ��(��ѧʽ��H2B��ʾ)��ˮ�еĵ��뷽��ʽ�ǣ�H2B===H����HB����HB��H����B2����
(5)��0.1 mol��L��1��Na2B��Һ�У���������Ũ�ȹ�ϵʽ��ȷ����_____ ___��
___��
A��c(B2��)��c(HB��)��0.1 mol��L��1
B��c(B2��)��c(HB��)��c(H2B)��0.1 mol��L��1
C��c(OH��)��c (H��)��c(HB��)
(H��)��c(HB��)
D��c(Na��)��c(OH��)��c(H��)��c(HB��)
������(1)һԪ��HA��NaOH�����ʵ�����Ӧ������ǿ��������ȫ�кͺ�����Һ��pH��a��7ʱΪǿ�ᣬa��7ʱΪ���ᡣ
(2)�ݵ���غ㣬��c(Na��)��c(H��)��c(A��)��c(OH��)����c(H��)��c(OH��)������c(Na��)��c(A��)��
(3)��Ϊ��Ũ�ȵ�HA��NaA�Ļ����Һ����pH��7֪A��ˮ��̶ȴ���HA�ĵ��룬����Ũ�ȴ�С��ϵΪc(Na��)��c(A��)��c(OH��)��c(H��)��
(4)�ݵ���غ�c(Na��)��c(H��)��c(A��)��c(OH��)���Ƶ�c(Na��)��c(A��)��c(OH��)��c(H��)��10��4��10��10 mol��L ��1��
��1��
(5)ע������еĵ��뷽��ʽ��һ������Ϊ��ȫ���롣�ж�A��ΪBԪ�ص������غ㣬C��Ϊ��Һ�е������غ㡣
�𰸡�(1)a��7ʱ��HA��ǿ�a��7ʱ��HA�����ᡡ
(2)C��(3)c(Na��)��c(A��)��c(OH��)��c(H��)
(4)10��4��10��10��(5)AC


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ�м��������������ʻ�ı������������ܴٽ�ˮ�ĵ��룬����ʹ��Һ��c(OH��)��c(H��)�IJ�����
��ϡ���ᡡ�ڽ����ơ��۰�������FeCl3���� ��NaClO���塡��ˮ�������
A���ڢ� B���٢�
C���ۢܢ� D����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й�������˵������ȷ������������ �� ��
A.���ǽ���Ԫ���ڵؿ��к�������Ԫ��
B.���DZȽϻ��õĽ������ڻ�ѧ��Ӧ������ʧȥ���ӣ����ֻ�ԭ��
C.�����ڿ��������ȿ����ۻ����ҷ������ҵ�ȼ��
D.�����ڿ��������ȿ����ۻ�������������Ĥ���ڣ��ۻ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��ݷ�Ӧԭ����Ƶ�Ӧ�ã�����ȷ����
A��CO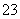 ��H2O
��H2O HCO
HCO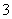 ��OH�������ȵĴ�����Һ��ϴ����
��OH�������ȵĴ�����Һ��ϴ����
B��Al3����3H2O Al(OH)3(����)��3H����������ˮ
Al(OH)3(����)��3H����������ˮ
C��TiCl4��(x��2)H2O(����)  TiO2��xH2O����4HCl���Ʊ�TiO2����
TiO2��xH2O����4HCl���Ʊ�TiO2����
D��SnCl2��H2O Sn(OH)Cl����HCl�������Ȼ�������Һʱ������������
Sn(OH)Cl����HCl�������Ȼ�������Һʱ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ʵ���Ũ�ȡ��������������Һ�У���H2CO3����Na2CO3����NaHCO3����NH4HCO3����(NH4)2CO3�����й�ϵ����˵����ȷ����
A��c(CO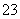 )�Ĵ�С��ϵΪ���ڣ��ݣ��ۣ��ܣ���
)�Ĵ�С��ϵΪ���ڣ��ݣ��ۣ��ܣ���
B��c(HCO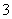 )�Ĵ�С��ϵΪ���ܣ��ۣ��ݣ��ڣ���
)�Ĵ�С��ϵΪ���ܣ��ۣ��ݣ��ڣ���
C������Һ��������ֻ�Тٲ��ܵõ���Ӧ�Ĺ�������
D���ڢۢܢݼ��������ᷴӦ��������NaOH��Һ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϻ�ʵ�����ᴿ�������ʵķ�������������(������Ϊ����)
A���屽(��)����NaOH��Һ����Һ
B��MgCl2��Һ(Fe3��)����MgO���壬����
C������(ˮ)����������ʯ�ң�����
D����������(���ᣬ�Ҵ�)���ӱ���̼������Һ����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����л��������о������������ʣ���ȥ��Щ���ʵķ�������ȷ����
A�����к��������ʣ�������ˮ������
B���Ҵ��к��������ʣ�����̼������Һϴ�ӣ���Һ
C����ȩ�к��������ʣ���������������Һϴ�ӣ���Һ
D�����ᶡ���к��������ʣ�����̼������Һϴ�ӣ���Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����(����ʽC4H10)�㷺Ӧ���ڼ���Һ��ʯ������Ҳ���ڴ�������ȼ�ϣ����й��ڶ�����������ȷ����
A���ڳ����£�C4H10������
B��C4H10��CH4��Ϊͬϵ��
C�����������������춡������ͬ���칹��
D��C4H10����һ��ȡ�����������ַе㲻ͬ�IJ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�Na2CO3��NaHCO3���ʱȽ��У���ȷ���ǣ� ��
A�������ȶ��ԣ�Na2CO3��NaHCO3 B������ʱˮ���ԣ�Na2CO3��NaHCO3
C����ϡ���ᷴӦ�Ŀ�����Na2CO3��NaHCO3D�����Ħ��������Na2CO3�� NaHCO3
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com