
��12�֣�
A.ԭ������ͬ������������ͬ�ķ��ӣ�����Ϊ�ȵ����塣
��.��֪A��B��C��D��E���ַ�������ԭ�ӵ���Ŀ����Ϊ1��2��3��6��6���Ҷ�����18�����ӣ���֪B��C��D��������Ԫ�ص�ԭ����ɣ���D����������ԭ�Ӹ�����Ϊ1 :2��
��ش�
��1�����A���ӵ�ԭ�ӵ�Ԫ�ط����� ����֪E���ж����л��E���ۡ��е��CH4���ۡ��е�ߣ�����Ҫԭ����____________________________________��
��2��C������ṹ�� ____ �Σ��÷������� ���ӣ�����ԡ��Ǽ��ԡ�����
��3������пɳ���������������D��Ϊȼ�Ϸ�Ӧ���ɵ�����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ_______ __________________ ��������Ҫд��Ӧ������
��.CO��N2��Ϊ�ȵ����塣
��4��CO���ܼ��ܴ���N2���ܼ��ܣ���CO��N2���ײμӻ�ѧ��Ӧ��
�����±����ݣ�˵��CO��N2���õ�ԭ����____________________________________��
|
|
|
A��B |
A=B |
A��B |
|
CO |
���ܣ�kJ/mol�� |
357.7 |
798.9 |
1071.9 |
|
���ܲ�ֵkJ/mol�� |
441.2 273 |
|||
|
N2 |
���ܣ�kJ/mol�� |
154.8 |
418.4 |
941.7 |
|
���ܲ�ֵkJ/mol�� |
263.6 523.3 |
��5�����ǵķ����ж�����___________���Ҽ���______________���м���
��6��Fe��Co��Ni�Ƚ�������CO��Ӧ��ԭ������Щ����ԭ�ӵĵ��Ӳ�ṹ�йء�
Niԭ�ӵļ۵����Ų�ʽΪ _____ ��Fe(CO)5�����³�Һ̬���۵�Ϊ
��20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe(CO)5�������� ____��������ͣ���Fe(CO)5������������__________ ��
B�����к��ж������������彡���ijɷ֣��ݲⶨ��Ҷ�к���450�����ϵ��л��ɷ���15�����ϵ�Ԫ�ء�ij��ѧ�о�С����̽����Ҷ�и�Ԫ�صĺ����������̽��ʵ�鷽�����£�����֪��Ҷ�е�������Ԫ�ضԸ����ӵIJⶨ��Ӱ�죩
����1����ȡ500g����IJ�Ҷ������ͨ����У��������ʹ��Ҷ�һ��������в�ĥϸ�������ձ��У�Ȼ��200mL 1 mol��L-1���������н��衢���ˡ�ϴ�ӡ�
����2������1������Һ����μ���ϡ����������Һ��������Һ��pH��6��7���ң�ʹ������Ԫ���������������ʽ��ȫ�������ټ������30 min������7.95g��ˮ̼���ƣ���ֽ��裬��������ȫ���ˣ�ϴ�ӣ����˺�õ���Һ�ͳ�����
����3��������2���õ���Һϡ����500 mL��ȡ���е�20.00 mL��Һ�Լ�����ָʾ������0.100mol��L-1��HCl����Һ�ζ����յ�ʱ������������Ϊ20.00mL����������
��ش��������⣺
���� 1�У�ʹ��Ҷ�һ�ʱ��Ҫ�õ����Ǽܡ������ǡ��ƾ���ơ� ___ �� ____ ��������
����2�У������Լ� _______ (д�Լ�����)������pH����Ϊ���㣻�жϳ����Ѿ�ϴ���ķ����� ��
����3�У��ζ������У��۾�Ӧע�� ____________________�����ζ���20 mL��Һ�к�CO32�������ʵ���Ϊ __ mol���Լ���ԭ500g��Ҷ�и����ӵ���������Ϊ _______ ������������£�
A.��1��Ar ��1�֣� E��CH3OH��CH3OH�γɷ��Ӽ������1�֣�
��2��V�Σ�����λ����������𰸣�����1�֣����Է��ӡ���1�֣�
��3��N2O4 + 2N2H4 = 3N2 + 4H2O��2�֣�
��4��CO�е�һ���м��ļ��ܱ�N2��С�ܶ࣬CO�ĵ�һ�������ϡ���1�֣�
��5��1��2��2�֣�
��6��3d84s2��1�֣������Ӿ��壨1�֣���CO��1�֣���
B������1������������ǯ��ÿ��1��,��2�֣�
����2����ˮ��2�֣�
ȡ���һ��ϴ��Һ�������Թ��У��μ�CaCl2��Һ�����������Ĵ𰸵Ⱦ��ɣ�������������������ϴ������2�֣����������𰸾����֣�
����3����ƿ����Һ��ɫ�仯�͵ζ�����Һ���������ʡ���2�֣�ֻ��ǰ��Ҳ���֣�
0.001 ��1�֣� 0.4%����0.004����1�֣�
������̣���2�֣�
����2���õ���Һ�к�CO32�������ʵ���Ϊ0.001mol��500��20=0.025mol
7.95g��ˮ̼���Ƶ����ʵ���Ϊ7.95g��106g/mol=0.075mol
ԭ500g��Ҷ�и�����(ʵ����ת��ΪCaCO3) �����ʵ���
=ת��ΪCaCO3��CO32�������ʵ���=0.075mol-0.025mol=0.050mol
ԭ500g��Ҷ�и����ӵ���������=0.050mol��40g/mol��500g=0.004
��������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
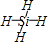
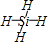
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��������«����һ����ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ���ѡ��
��֪��A��B��C��D��EΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�B�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ�ӵ���ͬ��B2�� ������ C2�����Ӿ�����ͬ���ȶ����Ӳ�ṹ��D�С����������֮�ƣ�D4�����Ӻ��ԭ�ӵĺ�������Ų���ͬ��E�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
��ش��������⣺
(1)A��B��C����Ԫ�صĵ縺���ɴ�С��˳����_______ _(��Ԫ�ط���)��
(2)��D�ĵ��ʾ����У�ԭ�ӵĶѻ���ʽ��Mgͬ������ѻ���ʽ�� ��Dԭ�ӵ���λ��������������
(3)����������������Դ��EԪ������(La)Ԫ�صĺϽ����������ϣ��úϽ�ľ�������ͼ��ʾ������������һ��Eԭ�ӣ�����Eԭ�Ӷ��ھ������ϣ���þ���Ļ�ѧʽΪ________����֪�þ�����ܶ�Ϊd g��cm��3���侧���ı߳�Ϊa cm��������ʵ�Ħ��������________��(����٤��������NA��ʾ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��������«����һ����ѧ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪��A��B��C��D��EΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�B�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ�ӵ���ͬ��B2�� ������ C2�����Ӿ�����ͬ���ȶ����Ӳ�ṹ��D�С����������֮�ƣ�D4�����Ӻ��ԭ�ӵĺ�������Ų���ͬ��E�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
��ش��������⣺
(1)A��B��C����Ԫ�صĵ縺���ɴ�С��˳����_______ _(��Ԫ�ط���)��
(2)��D�ĵ��ʾ����У�ԭ�ӵĶѻ���ʽ��Mgͬ������ѻ���ʽ�� ��Dԭ�ӵ���λ��������������

(3)����������������Դ��EԪ������(La)Ԫ�صĺϽ����������ϣ��úϽ�ľ�������ͼ��ʾ������������һ��Eԭ�ӣ�����Eԭ�Ӷ��ھ������ϣ���þ���Ļ�ѧʽΪ________����֪�þ�����ܶ�Ϊd g��cm��3���侧���ı߳�Ϊa cm��������ʵ�Ħ��������________��(����٤��������NA��ʾ)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com