
����Ŀ������������Ҫ���л��ϳ��м��塣������ij�о�С��ϳ��������·�ߣ�
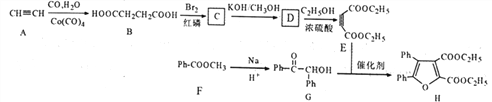
��֪����ph��Ϊ����
�� 
�ش��������⣺
(1)���������ڲⶨH�ṹ��������___________ ��������ţ�
a.�˴Ź����� b.��������� c.������
(2)G�й���������Ϊ__________��
(3)E�Ļ�ѧ������_______��B����C�ķ�Ӧ����Ϊ_______________��
(4)д����D����E�Ļ�ѧ����ʽΪ___________________��
(5)�����Ȼ�������������������������ֱ��������G��ͬ���칹�干��_____�֣���������
�ṹ�������к˴Ź���������ʾΪ5��壬�������Ϊ4:4:2:1:1����_____(д�ṹ��ʽ)��
(6)���������ϳ�·�ߣ���E���Ҵ�Ϊԭ��(���Լ���ѡ��������Ʊ�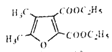 �ϳ�·��_______________________��
�ϳ�·��_______________________��
���𰸡� ab �ʻ����ǻ� ��Ȳ��������� ȡ����Ӧ HOOCC![]() CCOOH+2CH3CH2OH
CCOOH+2CH3CH2OH![]() CH3CH2OOCC
CH3CH2OOCC![]() CCOOCH2CH3 4
CCOOCH2CH3 4 ![]()
![]()
��������(1)a���˴Ź������ܲ���л�������ԭ���ӵ������Լ���Ŀ֮�ȣ��������⣬��a��ȷ��b�����ڼ���л�������������ż�������������Ҫ�����ڶ��Է����л�������ṹ���������⣬��b��ȷ��c���������ܲ���л�����Է������������������⣬��c���ʴ�Ϊab��
(2)![]() �й���������Ϊ�ʻ����ǻ���
�й���������Ϊ�ʻ����ǻ���
(3)![]() �Ļ�ѧ�����Ƕ�Ȳ�����������
�Ļ�ѧ�����Ƕ�Ȳ�����������![]() �ڴ�����������Br2����HOOCHBrCHBrCOOH�ķ�Ӧ����Ϊȡ����Ӧ��
�ڴ�����������Br2����HOOCHBrCHBrCOOH�ķ�Ӧ����Ϊȡ����Ӧ��
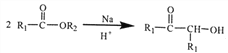
(4)��HOOCC![]() CCOOH���Ҵ�����������Ӧ����CH3CH2OOCC
CCOOH���Ҵ�����������Ӧ����CH3CH2OOCC![]() CCOOCH2CH3�Ļ�ѧ����ʽΪHOOCC
CCOOCH2CH3�Ļ�ѧ����ʽΪHOOCC![]() CCOOH+2CH3CH2OH
CCOOH+2CH3CH2OH![]() CH3CH2OOCC
CH3CH2OOCC![]() CCOOCH2CH3��
CCOOCH2CH3��
(5)�����Ȼ�������������������������ֱ�ӣ������![]() �ṹ������COOH�����ӷ�ʽ��4�֣�������������
�ṹ������COOH�����ӷ�ʽ��4�֣�������������![]() ͬ���칹����4�֣����к˴Ź���������ʾΪ5��壬�������Ϊ4:4:2:1:1����
ͬ���칹����4�֣����к˴Ź���������ʾΪ5��壬�������Ϊ4:4:2:1:1����![]() ��
��
(6)��![]() ���Ҵ�Ϊԭ���Ʊ�
���Ҵ�Ϊԭ���Ʊ�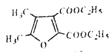 �ϳ�·��Ϊ
�ϳ�·��Ϊ![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵�������ε���������Aԭ�Ӻ���������δ�ɶԵ��ӣ�������B2EΪ���Ӿ��壬Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ���������ͬ���ڵĵ�����û����ͬ�ģ�Fԭ��������������B����ͬ�����������������ӡ������������Ϣ���ش��������⣨����ʱ��A��B��C��D��E��F������Ӧ��Ԫ�ط��ű�ʾ����
��1��A��B��C��D����Ԫ�ص�һ������������_______________��
��2��B���Ȼ�����۵��D���Ȼ�����۵�__________(����������������)��������
__________________________________________________��
��3��E�ĵͼ���������ӵ����幹����_________________����KMnO4������Һ���ո�������ʱ��MnO4-����ԭΪMn2+���÷�Ӧ�����ӷ���ʽΪ______________________________________��
��4��F�ĺ�������Ų�ʽ��___________��
��5��A��F�γ�ij�ֻ�����ľ����ṹ����ͼ��ʾ������A��-3�ۣ����û�����Ļ�ѧʽ��_________________���������߳�Ϊa cm������٤������ΪNA����þ�����ܶȼ���ʽΪ��=___________g/cm3���ú�a��NA�ķ��ű�ʾ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̫���ܵ����ͨ�����ЧӦ���߹⻯ѧЧӦֱ�Ӱѹ���ת���ɵ��ܵ�װ�á�������е����裬����ͭ���ࡢ�ء����Ȼ����
��1����ͭ����(Cu+)��̬ʱ�����Ų�ʽΪ_______________���������ռ�ݵ�ԭ�ӹ����ĿΪ___________����
��2������ͼ��ʾ̼�������Ԫ�ص��ļ������ܱ仯���ƣ����б�ʾ��������_______________������a��b��c����

��3����������ɶ��������Ƶã��������辧��ṹ����ͼ��ʾ���ڶ������辧���У�Si�� Oԭ�������ӵ���С��Ϊʮ��Ԫ������ÿ��Siԭ������ _________��ʮ��Ԫ����
��4��������(GaN)�ľ���ṹ����ͼ��ʾ����ѹ�£��þ����� ��1700�棬���侧������Ϊ_______���жϸþ���ṹ�д�����λ����������________________��
��5������Ԫ�ش���ͬһ�������Ԫ�ؾ���ȱ�����ԣ��������(H3BO3)��ˮ��Һ������ˮ��Ӧ����[B(OH)4]-��[B(OH)4]-��Bԭ�ӵ��ӻ��������Ϊ________________�������ǿռ乹�ͣ�[B(OH)4]-��ԭ�ӵijɼ���ʽ�ýṹ��ʽ��ʾΪ_______________________��
��6��ij�������������������ͭ�������ﰴһ�������ۺ϶��ɣ�������������ᄃ���ṹ����ͼ��ʾ�������ʵĻ�ѧʽΪ________����֪�þ� ���ܶ�Ϊ��g��cm-3�������߳�Ϊa pm����������ԭ������Ϊ_____________���ú��Ѻ�a�Ĺ�ϵʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������⣬Υ����ѧ�仯���ɵ��ǣ� ��
A. ʯī�Ƴɽ��ʯB. ú����������ʯ��
C. ˮ�������D. ��ˮ�������Ի��ˮΣ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ĥ��������ƿ��ŵ��Լ��У� ��
A. �ռ���Һ B. ����� C. Ũ���� D. ��������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������л���ķ����������ǻ������ŵķ�Ӧ�����У�(a)������Ӧ��(b)ȡ����Ӧ��(c)��ȥ��Ӧ��(d)�ӳɷ�Ӧ��(e)ˮ�ⷴӦ��������ȷ�������
A��(a)(b)(c) B.(d)(e)
C.(b)(d)(e) D��(b)(c)(d)(e)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʼ��ÿ��ת������ͨ��һ����Ӧ����ʵ�ֵ���
A. MgCl2��Mg��Mg3N2 B. Al2O3��Al��OH��3��NaAlO2
C. S��SO2��H2SO4 D. NaCl��Cl2��FeCl3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ƶ�ʵ�鷽���ܴﵽ��Ӧʵ��Ŀ������������
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
A | �����ʷ����˱��� | ������Һ�м���CuCl2��(NH4)2SO4������Һ |
B | ֤����Ӧ���ʻ��淴Ӧ��Ũ�ȵ�������ӿ� | ��3mLϡ������������п��Ӧ�������������ʽ�����Ȼ�����1mL 1mol��L��1CuSO4��Һ��Ѹ�ٲ����϶����� |
C | �Ƚ�Ksp(BaCO3)��Ksp(BaSO4) | �����£���Na2CO3��Һ�м�����BaSO4��ĩ�����ˣ���ϴ���ij����м�ϡ���ᣬ�����ݲ��� |
D | ͨ���۲�Һ����жϸ�װ�õ������� |
|
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com