
������Ũ�ȶ�Ϊ0.1 mol/L��������Һ�У����루��ͨ�룩ij���ʺ�����Ӧ�Ⱥ�˳����ȷ����
| A���ں�Fe3+��Cu2+��H+����Һ�м���п�ۣ�Cu2+��Fe3+��H+ |
| B���ں�I����SO32����Br������Һ�в���ͨ��������I����Br����SO32�� |
| C���ں�Fe3+��H+��NH4+����Һ����μ����ռ���Һ��Fe3+��NH4+��H+ |
| D���ں�AlO2����SO32����OH������Һ����μ�������������Һ��OH����AlO2����SO32�� |
D
�������������A�������ԣ�Fe3+��Cu2+��H+������������ԭ��Ӧ���Ⱥ���ɣ�������Ӧ�Ⱥ�˳��Ϊ��Fe3+��Cu2+��H+����A����B����ԭ�ԣ�SO32-��I-��Br-������������ԭ��Ӧ���Ⱥ���ɣ�������Ӧ�Ⱥ�˳��Ϊ��SO32-��I-��Br-����B����C���ȶ��ԣ�ˮ������������һˮ�ϰ����ʷ�����Ӧ�Ⱥ�˳��Ϊ��H+��Fe3+��NH4+����C����D�����ԣ�OH-��AlO2-��SO32-���ʷ�����Ӧ�Ⱥ�˳��Ϊ��OH-��AlO2-��SO32-����D��ȷ����ѡD��
���㣺���������ԡ���ԭ��ǿ���Ƚϣ����ӷ�Ӧ����������


 ȫ��������ϵ�д�
ȫ��������ϵ�д� һ��һ����ʱ���ϵ�д�
һ��һ����ʱ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����β������ɴ�����Ⱦ����Ҫԭ��֮һ���������������ϰ�װ����ת������ʹ������β��ת������������Ŀǰ����Ч���ֶ�֮һ������� ��ʾ̼ԭ�ӣ���
��ʾ̼ԭ�ӣ��� ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ��� ��ʾ��ԭ�ӣ���ͼΪ����ת�����۹��̡��������ͼʾ�ش��������⣺
��ʾ��ԭ�ӣ���ͼΪ����ת�����۹��̡��������ͼʾ�ش��������⣺
(1)A��B��C�������ʿ��Թ�Ϊһ��������� ��
(2)��C��Ϊ�������D��Ϊ���ʵ������� ��
(3)�û�ѧ��Ӧ����ʽ��ʾΪ ��
��ѧ�仯���������ĵ�A���ʺ����ɵ�C���ʵ�������Ϊ ��
(4)���۵ĽǶ�ȥ�������õĹ��ڻ�ѧ�仯���й���Ϣ(���һ������) ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������������Ҫ�Ľ��������ǵĵ��ʼ��仯�������Ÿ��Ե����ʡ�
��1��������100mL 0.01mol?L��1 FeCl3��Һ�����ƹ�������Ҫ�IJ�����������Ͳ����ͷ�ιܡ��ձ��⣬����Ҫ �����ƹ����г�����FeCl3���塢����ˮ�⣬����Ҫ���Լ��� ���������ƺõ���Һ�����ˮ�в����һ��ʱ�䣬�ɵõ����ɫҺ�壬�÷�Ӧ�����ӷ���ʽΪ ����Һ����е������� ������ĸ����
a������ͨ����Һ��ʱ�γɹ����ġ�ͨ·��
b�����Һ���м���AgNO3��Һ����������
c������Һ����й��ˣ��ɵõ����ɫ����
d������Һ����ȡ����ɡ����պ�������������
��2���ڸ��������������£���þ����ȼFe3O4��ĩ�����۵ľ��Ȼ���ʹ���ַ�Ӧ��
�����ַ�Ӧ���ʣ������м���������NaOH��Һ���д������ݲ���������ʣ������г�������еĹ��������� ���ѧʽ����
����֪��3Fe(s)+2O2(g)=Fe3O4(s) ��H��?1118 kJ·mol��1
2Al(s)+3/2O2(g)=Al2O3(s) ��H��?1675.7 kJ·mol��1
������Fe3O4������Ӧ��������Al2O3���Ȼ�ѧ����ʽΪ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͭ�������ڿ����л�Ϳ����е�ˮ������CO2��O2���ò��������⡱���á����⡱�׳ơ�ͭ�̡����ֳơ���ȸʯ��[��ѧʽΪCu2(OH)2CO3]����ͭ�̡��ܸ��ᷴӦ����ͭ�Ρ�CO2��H2O��ijͬѧ��������ϵ�з�Ӧʵ���ˡ�ͭ��ͭ�̡�������ͭ����ת����
��1�������ֲ�ͬ������ش𣬡�ͭ�̡������������ʣ�___________________________��
��2����д���ڴ������ӷ���ʽ��______________________________________________________________��
��3������ת������������������ԭ��Ӧ����_________������ţ���ͬ�������ڸ��ֽⷴӦ����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��11�֣����෨�ǻ�ѧѧϰ��һ��ʮ����Ч�ķ�����������ʹ���ǴӲ�ͬ�Ƕȶ�ͬһ֪ʶ������������˽⣬���Ǹ��ݲ�ͬ�ı�����ʹͬһ����Ҳ���ܱ����ڲ�ͬ������С������������ʢ�MgCl2��HCl ��SO2 �� NO2 ��K2CO3 �� Cl2 ��CaO �� NaOH
��������б������������ʽ��з��ࣺ
��1���Ⱥ����Ӽ��ֺ����ۼ��������� ������ţ���ͬ����
��2��ֻ�����ۼ���Ϊ�ǵ���ʵ��� ��
��3�����й��ۼ��Ļ������� ��
����ij���ݵ��ܱ������м���A��B��C�������壬��ͼ��ʾ��һ��������������������ʵ�����ʱ��ı仯�����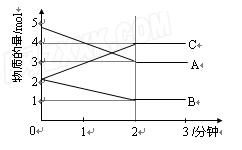
��1��д�����ܱ�����������Ӧ�Ļ�ѧ����ʽΪ ��
��2��2���Ӻ�A��B��C�����ʵ���������ʱ���
�仯���仯����ԭ����____ ��
��3�����������˵���÷�Ӧ�ﵽ��ѧ��Ӧ�ȵ��� ��
A����������ѹǿ���ٷ����仯��
B����λʱ��������A�����ʵ���������C�����ʵ�����ͬ��
C����������������ܶȲ��ٸı䣻
D��������A�����ʵ�����C�����ʵ�����ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���и�ѡ���е�������Ӧ,����ͬһ�����ӷ���ʽ��ʾ���� ( )
| ѡ�� | �� | �� |
| A | Ba(OH)2��Һ�����NaHCO3��Һ��� | NaOH��Һ�����NaHCO3��Һ��� |
| B | ����SO2ͨ��Ba(OH)2��Һ�� | ����SO2ͨ��Ba(OH)2��Һ�� |
| C | BaCl2��Һ��Na2SO3��Һ��� | Ba(OH)2��Һ��H2SO3��Һ��� |
| D | ������ˮ����AlCl3��Һ�� | ����AlCl3��Һ���백ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������ӷ���ʽ��ѧ����ʽ��д��ȷ���� �� ��
A��ʵ�������Ȼ������ʯ���ư��� NH4++OH�� NH3��+H2O NH3��+H2O |
| B����CO2ͨ��BaCl2��Һ�� H2O +CO2+Ba2+ =BaCO3��+2H+ |
| C������SO2ͨ���Ư�۵�ˮ��Һ�� SO2+H2O+Ca2++3ClO�� = CaSO4��+2HClO+Cl�� |
D����H2��ԭMgO H2+MgO  Mg+H2O Mg+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����£����и���������ָ����Һ��һ���ܴ����������
| A��0.1 mol��L��1 KNO3��Һ��Mg2+��Fe3+��Cl����SO42�� |
| B����ɫ������Һ��Cu2+��NH4����NO3����CO32�� |
| C��ʹpH��ֽ������Һ��K����Ca2+��Cl����ClO�� |
| D��ˮ�������c(H��)=10��12 mol��L��1����Һ��Na����NH4����SO42����NO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����йر�����ȷ����
| A�������������ʹ��ѧƽ���ƶ� |
| B���ѷ���NO2����ƿ������ˮ�У���ɫ��dz |
| C����30%��FeCl3��Һ��ʴӡˢ��·���ϵ�ͭ����Fe3+ + Cu=Fe2+ + Cu2+ |
| D��������NaHSO4��Ba(OH)2��Һ��Ӧ��Ba2++2OH��+2H++SO42��=BaSO4��+2H2O |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com