
����β������ɴ�����Ⱦ����Ҫԭ��֮һ���������������ϰ�װ����ת������ʹ������β��ת������������Ŀǰ����Ч���ֶ�֮һ������� ��ʾ̼ԭ�ӣ���
��ʾ̼ԭ�ӣ��� ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ��� ��ʾ��ԭ�ӣ���ͼΪ����ת�����۹��̡��������ͼʾ�ش��������⣺
��ʾ��ԭ�ӣ���ͼΪ����ת�����۹��̡��������ͼʾ�ش��������⣺
(1)A��B��C�������ʿ��Թ�Ϊһ��������� ��
(2)��C��Ϊ�������D��Ϊ���ʵ������� ��
(3)�û�ѧ��Ӧ����ʽ��ʾΪ ��
��ѧ�仯���������ĵ�A���ʺ����ɵ�C���ʵ�������Ϊ ��
(4)���۵ĽǶ�ȥ�������õĹ��ڻ�ѧ�仯���й���Ϣ(���һ������) ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���й������ʷ������ȷ����ǣ� ��
| ��� ���� | �� | �� | �� | ���������� | ���������� |
| A | ���� | ���� | �ռ� | ������ | �������� |
| B | �ռ� | ���� | ʳ�� | ������ | �������� |
| C | ������ | ʯ̿�� | ����� | �������� | �������� |
| D | ���Լ� | Ӳ֬�� | С�մ� | ������ | ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����˵����ȷ����
A����֪KCl��MgO�ľ���ṹ��NaCl�ľ���ṹ���ƣ�������۵㣺MgO>KCl>NaCl
B ����з�̪��̼������Һ�м���BaCl2��Һ����Һ��ɫ
C����ɢϵ�з�ɢ�����ӵ�ֱ����Fe(OH)3����>Fe(OH)3����Һ>FeCl3��Һ
D��Na2O��MgO��Al2O3�����ڼ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������ڻ�ѧѧ�Ƶķ�չ�����˷dz���Ҫ�����á�ͼ1��ij��Ӧ���ܱ������з�Ӧǰ��ķ���״��ʾ��ͼ���� ���͡�
���͡� ���ֱ��ʾ��ͬ��ԭ�ӡ��Դ˷�Ӧ�ķ���һ������ȷ����
���ֱ��ʾ��ͬ��ԭ�ӡ��Դ˷�Ӧ�ķ���һ������ȷ����
| A���û���Ӧ | B��������ԭ��Ӧ | C�����ȷ�Ӧ | D�����Ϸ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��17�֣��������������ʣ��پƾ�����ͭ���������������ܰ����������ǡ� �����ᡢ��̼�����ơ������ᡢ�����⡢��Al2(SO4)3���������ʵ������д���пհ�
��1������ǿ����ʵ��У� ��
��2��Һ̬ʱ�ܵ�����Ϊ�����仯���У� ��
��3������ˮ��Һ�ĵ��뷽��ʽΪ ��
��A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʡ�����֮�������µķ�Ӧ��ϵ��
��1����A�ǵ���ɫ���壬C��D���������C������������Ҫ���ʡ�����C���ʿɵõ��м�ֵ�Ļ�ѧƷ��д���û�ѧƷ�е�1�����1���ε������� ������������ ��������
��2����B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ��B��C��һ�������·�Ӧ���ɵ�A�Ǵ�����Ҫ�ɷ֣�д���÷�Ӧ�Ļ�ѧ����ʽ������������ �� ������ ����������
��3����D���ʾ������ԣ��ڢ۷�Ӧ��Ҫ��ǿ����Һ���ܷ�Ӧ��ͨ�������һ����������ЧӦ����Ҫ���塣�жϵ���A��Ԫ�������ڱ��е�λ����___ _____��д�ܷ�Ӧ���ӷ�����������
��4����A��Ӧ����㷺�Ľ������ܷ�Ӧ�õ�A���ڢݷ�Ӧ���õ�ͬһ�ַǽ������ʡ�C����Һ����ʴ��ӡˢͭ��·�壬д�÷�Ӧ�����ӷ���ʽ����������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ɸ�������ɺ����ʽ��з��ࡣ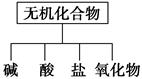
(1)��ͼ��ʾ�����ʷ������ ��
(2)��Na��K��H��O��C��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��ڢۢĺ��档
| ������� | �� | �� | �� | ������ |
| ��ѧʽ | ��HCl�� | �� ��Ba(OH)2 | ��Na2CO3�� | ��CO2 ��Na2O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
NH4Al(SO4)2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ�У�NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش��������⣺
��1��NH4Al(SO4)2������ˮ������������ (�ñ�Ҫ�Ļ�ѧ������������˵��)��
��2����ͬ�����£�0��1 mol��L��1NH4Al(SO4)2��c(NH4+) (����ڡ��������ڡ���С�ڡ�)0��1 mol��L��1NH4HSO4��c(NH4+)��
��3��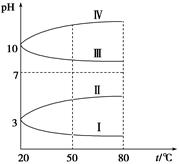
��ͼ��0��1 mol��L��1�������Һ��pH���¶ȱ仯��ͼ��
�����з���0��1 mol��L��1NH4Al(SO4)2��pH���¶ȱ仯�������� (��д��ĸ)������pH���¶ȱ仯��ԭ���� ��
��20 ��ʱ��0��1 mol��L��1NH4Al(SO4)2��2c(SO42-)��c(NH4+)��3c(Al3��)�� ��
��4������ʱ����100 mL 0��1 mol��L��1NH4HSO4��Һ�еμ�0��1 mol��L��1NaOH��Һ���õ���ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ��
�Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶������� ����b�㣬��Һ�и�����Ũ���ɴ�С������˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��ֻ��һ���Լ����ɳ�ȥ�������ʺͼ������ʡ��������ڿո�
| ��� | ���� | �Լ����ƻ�ѧʽ |
| �� | �����ʣ�NaHCO3��Һ��Na2CO3�� | |
| �� | �����ʣ�SiO2��CaCO3�� | |
| �� | �����ʣ�FeCl2��Һ��FeCl3�� | |
| �� | ����Na2CO3 Na2SiO3 Na2SO3��Һ | |
| �� | ���𣺣�NH4��2SO4 NH4C1 Na2SO4��Һ | |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������Ũ�ȶ�Ϊ0.1 mol/L��������Һ�У����루��ͨ�룩ij���ʺ�����Ӧ�Ⱥ�˳����ȷ����
| A���ں�Fe3+��Cu2+��H+����Һ�м���п�ۣ�Cu2+��Fe3+��H+ |
| B���ں�I����SO32����Br������Һ�в���ͨ��������I����Br����SO32�� |
| C���ں�Fe3+��H+��NH4+����Һ����μ����ռ���Һ��Fe3+��NH4+��H+ |
| D���ں�AlO2����SO32����OH������Һ����μ�������������Һ��OH����AlO2����SO32�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com