
��������ɸ�������ɺ����ʽ��з��ࡣ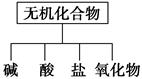
(1)��ͼ��ʾ�����ʷ������ ��
(2)��Na��K��H��O��C��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��ڢۢĺ��档
| ������� | �� | �� | �� | ������ |
| ��ѧʽ | ��HCl�� | �� ��Ba(OH)2 | ��Na2CO3�� | ��CO2 ��Na2O2 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������ѧϰ���о���ѧ��һ�ֵ���Ҫ���������з����������
| A��K2CO3��K2O�������� | B��H2SO4��HNO3�������� |
| C��KOH��Na2CO3�����ڼ� | D��Na2O��Na2SiO3������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����ǻ�ѧ�о��г��õķ��������з�����У���ȷ���� ( )
| A��SO2��SiO2��CO������������ |
| B��ϡ���������ᡢ�Ȼ�����Һ��Ϊ���� |
| C���ռ�����ᡢ���Ȼ�̼��Ϊ����� |
| D���������֡�ˮ��������ˮ��Ϊ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�ѡ�������գ�
��16O��18O �ڽ��ʯ��ʯī �� CH3CH3��CH3CH2CH2CH3
��CH3CH2CH2CH3 ��CH3CH (CH3)CH3
��1������ͬ����������� �� ��2����Ϊͬλ�ص��� ��
��3����Ϊͬ���칹����� �� ��4������ͬϵ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����β������ɴ�����Ⱦ����Ҫԭ��֮һ���������������ϰ�װ����ת������ʹ������β��ת������������Ŀǰ����Ч���ֶ�֮һ������� ��ʾ̼ԭ�ӣ���
��ʾ̼ԭ�ӣ��� ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ��� ��ʾ��ԭ�ӣ���ͼΪ����ת�����۹��̡��������ͼʾ�ش��������⣺
��ʾ��ԭ�ӣ���ͼΪ����ת�����۹��̡��������ͼʾ�ش��������⣺
(1)A��B��C�������ʿ��Թ�Ϊһ��������� ��
(2)��C��Ϊ�������D��Ϊ���ʵ������� ��
(3)�û�ѧ��Ӧ����ʽ��ʾΪ ��
��ѧ�仯���������ĵ�A���ʺ����ɵ�C���ʵ�������Ϊ ��
(4)���۵ĽǶ�ȥ�������õĹ��ڻ�ѧ�仯���й���Ϣ(���һ������) ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ͼ��ʾ���й�����ת����ϵ�У������ʾ���������ѧ��ѧ��ѧ�����ʡ�CΪһ�ֺ�ɫ��ĩ��DΪһ�����塣
����ݿ�ͼ�����ʵ�ת����ϵ�������Ϣ���û�ѧʽ��д���пհף�
(1)��FΪ�����ԼGΪ��ɫ��������AΪ ��D����Ϊ ��
(2)��GΪ��ɫ������HΪ�������Σ���IΪ ��
(3)��G��H�������Σ���GΪ��ɫ��Һ����I����Ϊ ��F����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������г��õ���������ʣ��뿴����������
(1)��ʵ��ʱ����ָ�������������ƣ��ɴӼ�������ȡ�Ȼ�����ҺӦ��ֹѪ����ԭ����
_______________________________________________��
(2)���մɹ�ҵ�ϳ������������������������Ӱ���Ʒ�������������֮һ�ǰ���Щ������ˮһ����裬ʹ��ֱ����10��9��10��7m֮�䣬Ȼ����������缫���ٽ�ֱͨ����Դ����ʱ�����ۼ����������������ۼ�������������������________��
(3)ˮ���ұ�����ø�ѹ������ܽ������Գ�ȥ�����̳������ٶԿ�������Ⱦ����������������________ԭ����
(4)������������ǿ������Һ����������η�Ӧ�����ɸ�������(FeO42��)��FeO42����ǿ�����ԣ�����ɱ���������������λ��о���ˮ�����ã��ܾ���ˮ��ԭ���� ______________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���෨��ѧϰ��ѧ����Ҫ��������������ʶ����ʱ�ɲ��ö��ַ�������±��������
| ��� | ����� | ���� |
| A | FeSO4��NO2��MnO2��NaClO��Cu3P��Na2O2 | H2SO3 |
| B | CH3COOH��HOOC��COOH��HClO��H2S | HF |
| C | ���ֽⷴӦ����ⷴӦ�����ȷ�Ӧ�����ӷ�Ӧ | ��ɫ��Ӧ |
| D | (NH4)2SO4��NH4Cl��NH4NO3��NH3��H2O | NH4HCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���и�ѡ���е�������Ӧ,����ͬһ�����ӷ���ʽ��ʾ���� ( )
| ѡ�� | �� | �� |
| A | Ba(OH)2��Һ�����NaHCO3��Һ��� | NaOH��Һ�����NaHCO3��Һ��� |
| B | ����SO2ͨ��Ba(OH)2��Һ�� | ����SO2ͨ��Ba(OH)2��Һ�� |
| C | BaCl2��Һ��Na2SO3��Һ��� | Ba(OH)2��Һ��H2SO3��Һ��� |
| D | ������ˮ����AlCl3��Һ�� | ����AlCl3��Һ���백ˮ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com