

| c(��)V(��) |
| V(����) |
| 0.005mol��248g/mol |
| 1.28g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
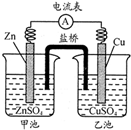 ��ѧ�̲������˴����ŵ�ԭ��أ��õ�ؼ�Ϊ1806�굤�����Ƶ�ԭ��أ���ͼ�������йط�����ȷ���ǣ�������
��ѧ�̲������˴����ŵ�ԭ��أ��õ�ؼ�Ϊ1806�굤�����Ƶ�ԭ��أ���ͼ�������йط�����ȷ���ǣ�������| A����������������Ӧ��Cu-2e-=Cu2+ |
| B����ع���ʱ���������� |
| C�����ҳ���ͨ��H2S��ط�Ӧֹͣ |
| D�������缫Cu��Ϊʯī����ص���ǿ�ȷ����仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��-332 | B��-118 |
| C��+350 | D��+130 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
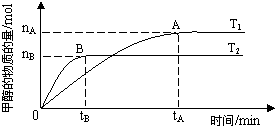
| n(H2) |
| n(CH3OH) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����������� | ʵ����� | �ж����� | |
| ����һ | �������NaOH��Һ���õ���ɫ������ | �϶���Cu2+���϶��� |
�϶������ӵ����� |
| ����� | ����ɫ�������ˡ�ϴ�ӡ����յõ�24.0g���壻 | Cu2+���ʵ���Ũ�� Ϊ |
CuԪ���غ� |
| ������ | ��������Һ�м�����Ba��NO3��2��Һ���õ�46.6g������ϡ����ij����� | �϶���Cl-�� �϶���Ba2+�� |
�϶���Cl-������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
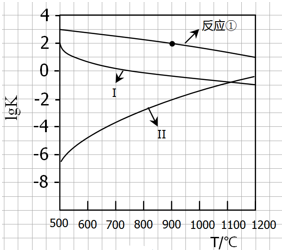 ��CaSO4����O2��ȼ��CO��Ӧ���ȿ����ȼ��Ч�ʣ����ܵõ��ߴ�CO2����һ�ָ�Ч����ࡢ���õ�����ȼ�ռ�������Ӧ��Ϊ����Ӧ����Ӧ�ں͢�Ϊ����Ӧ��
��CaSO4����O2��ȼ��CO��Ӧ���ȿ����ȼ��Ч�ʣ����ܵõ��ߴ�CO2����һ�ָ�Ч����ࡢ���õ�����ȼ�ռ�������Ӧ��Ϊ����Ӧ����Ӧ�ں͢�Ϊ����Ӧ��| 1 |
| 4 |
| 1 |
| 4 |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
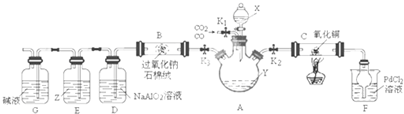
| ��� | ʵ����� | ʵ����������� | �� �� | ||
| �� | ����a g M�м���һ����ϡ���ᣬ��ֽ��裻 �ڼ����μ�ϡ��������������ַ�Ӧ�� |
�ٹ������Լ��٣� ����Ȼ��һ�������壬��Һ����ɫ |
��M��һ����Cu2O�� ��M��һ����Cu�� | ||
| �� | �ٽ���ʵ���������Һ���� �ڽ�����ϴ�ӡ�������� |
��������Ϊ
|
MΪCu��Cu2O�Ļ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com