��2011?��ɽ��ģ�⣩���������ϣ���������AlN���մ���һ������ʯ��������������ǽ������ϣ���߿��ȶ���2200�森�����Ժã�������ϵ��С�������õ����ȳ�����ϣ������ڽ�����ʴ������ǿ���������������������Ͻ�������������ϣ����������ǵ��Ե�壬����������ã���������Ԫ��Ҳ����ϣ������ϸ��������ĩ���㷺Ӧ���ڴ��ģ���ɵ�·������������ȡԭ��Ϊ��
Al
2O
3+3C+N
2 2AlN+3CO
������̽����ij��ѧ�о���ѧϰС���Ա���ݵ���������ȡԭ��������������̽����
����1������ȡ������ʱ���ڷ�Ӧ����ȫ����������Ʒ���������ʳ���̼������ܴ���
������
������
��
����2��Ϊ�ⶨ�ò�Ʒ���йسɷֵĺ������ס�����ͬѧ�������������ʵ�飺
��1����ͬѧ����ȡ10.00g��Ʒ������������������������Һ�й��Ȳ����ɣ�AlN������������Һ��Ӧ����NaAlO
2�����ų�����3.36L����״������
��������Ӧ�Ļ�ѧ����ʽΪ
AlN+NaOH+H2O=NaAlO2+NH3��
AlN+NaOH+H2O=NaAlO2+NH3��
��
�ڸ���Ʒ�е�AlN����������Ϊ
61.5%
61.5%
��
��2����ͬѧ����ȡ10.00g��Ʒ���ڷ�Ӧ���У�ͨ��2.016L����״����O
2���ڸ����³�ַ�Ӧ����������ܶ�Ϊ1.34g?L
-1�����۳ɱ�״����AlN����O
2��Ӧ��������Ʒ�к�����̼
1.92
1.92
g��
����3����ͬѧ�ܵ��ס���ͬѧʵ�����������Ϊ�ⶨij�������к���̼�����������ʣ�����ͼ��I��һЩװ�������м��飬����AlN��NaOH��Һ��Ӧ�����ɰ�����������ⶨ��Ʒ�е�����������������������ʵ��������ȷ�����ʵijɷ֣�ʵ���е���������Բ��ƣ�
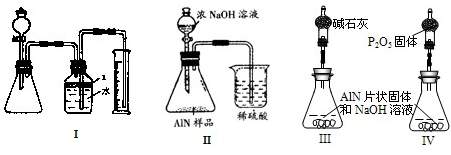
��1��ʵ���йز���Ϊ��������ƿ�з���������AlN��Ʒ���ڴӷ�Һ©������ƿ�м��������ŨNaOH���ۼ���װ�õ������ԣ��ܲⶨ�ռ���ˮ�������
��ȷ�IJ���˳��Ϊ
�ۢ٢ڢ�
�ۢ٢ڢ�
��
��2���������м��װ�������Եķ�����
�رշ�Һ©������������ƿ�����ƿ���Ҳർ��ˮ������������ʱˮ����������
�رշ�Һ©������������ƿ�����ƿ���Ҳർ��ˮ������������ʱˮ����������
��
��3�����ƿ�е��Լ�X�����ѡ��
C
C
����ѡ��ı�ţ���
A���� B���ƾ� C��ֲ���� D��CCl
4��4�����ƿ��Һ��û��װ�����Ϸ����������ռ䣩��ʵ����NH
3�������
����
����
����ƫ��ƫС�䣩��
��5����ʵ���в����Ʒ������Ϊwg�����������ΪaL������£�������Ʒ��AlN����������Ϊ
��AlN��ʽ��Ϊ41����
��6��ʵ����������۲쵽��ƿ�л��й��壬����Ʒ�к��е�������
̼
̼
��Ϊ�˲ⶨ�Ƿ����������ʣ�����Ҫ��Щ������
̼������
̼������
��
����4����ͬѧ��Ϊ����ͬѧ��ʵ�鷽��������������������������������ϴ������ͼ9�еĢ�װ�ý���ͬ��ʵ�飬ͨ���ⶨ�ձ��������������ȷ����Ʒ��AlN����������������Ϊ�Ƿ���У�
������
������
�����롰���С����������С�����ԭ����
II��NH3���ױ����գ�������������ͬʱ�����к���ˮ������Ӱ�찱�������IJⶨ
II��NH3���ױ����գ�������������ͬʱ�����к���ˮ������Ӱ�찱�������IJⶨ
����ĸĽ�����Ϊ
��װ��֮������ʢ�м�ʯ�ҵĸ���ܣ��ձ����ܵ�ĩ�˽�һ���۵�©�������հ���
��װ��֮������ʢ�м�ʯ�ҵĸ���ܣ��ձ����ܵ�ĩ�˽�һ���۵�©�������հ���
��
����5����ͬѧ��ϸ˼���˶�ͬѧ��װ�ú���Ϊ��װ�������õ���Ʒ��AlN����ƫС����ԭ����
��Ӧ�����İ��������ܱ���ȫ����
��Ӧ�����İ��������ܱ���ȫ����
�������Դ�ԭ��Ļ���ֻҪ��ͼ9�е�III��IV����װ���е�һ�֣�ֻ����м��ֱ�Ҫ�����ݲⶨ���ɱȽ�ȷ��ȷ����Ʒ��AlN�������������Ϻ�����װ��Ϊ
��
��
������ţ�������Ϊ��ͬѧ��װ���Ƿ���ȱ�ݣ�
��
��
�����У���������ƫ��ƫ��
ƫ��
ƫ��
��Ӧ����θĽ���
Ӧ�ٽ�һ����IVװ������ȫ��ͬ�ĸ���ܣ����ٽ�һ��װ�м�ʯ�ҵĸ����Ҳ�У�
Ӧ�ٽ�һ����IVװ������ȫ��ͬ�ĸ���ܣ����ٽ�һ��װ�м�ʯ�ҵĸ����Ҳ�У�
��������ȱ�ݺ����˸�ɲ����





 ��2011?��ɽ��ģ�⣩��������ȼ�ϵ�ع���ʱ�ɲ����������⣨��ͼ1����������̼��Carbon����������С�Բ������������Ũ�ȣ�concentration����Ӱ�죨��Ӱ��������ͼ2��ʾ����
��2011?��ɽ��ģ�⣩��������ȼ�ϵ�ع���ʱ�ɲ����������⣨��ͼ1����������̼��Carbon����������С�Բ������������Ũ�ȣ�concentration����Ӱ�죨��Ӱ��������ͼ2��ʾ����


 NH4++NH2-
NH4++NH2- NH4++NH2-
NH4++NH2- ��2��¯�����Ƶ������ǽ���SO2���г�����ˮϴ��������¯����SO2�Ĵ��������ڽӴ����н��У�
��2��¯�����Ƶ������ǽ���SO2���г�����ˮϴ��������¯����SO2�Ĵ��������ڽӴ����н��У�