
����Ŀ���ӻ�ͭ���н�ȡͭԪ�أ�����FeCl3����ȡ����
(1)��ӦCu2S+4FeCl3=2CuCl2+4FeCl2+S��ÿ����1mol CuCl2����Ӧ��ת�Ƶ��ӵ���ĿΪ_______����ȡʱ�������������¿�ά��Fe3+�ϸ�Ũ�ȡ��йط�Ӧ�����ӷ���ʽ��______________��
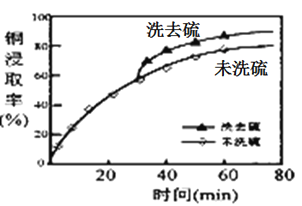
(2)��ȡ�����м���ϴ�Ӽ��ܽ���ʱ��ͭԪ�صĽ�ȡ�ʵı仯����ͼ����ԭ����_____________��
(3)353Kʱ����FeCl3��ȡҺ�м���CuCl2���ܼӿ�ͭԪ�صĽ�ȡ���ʣ��䷴Ӧԭ�����û�ѧ����ʽ��ʾΪ��________________________________________________________��CuCl+FeCl3=CuCl2+FeCl2��
(4)��ͭ����ɻ�ͭ��(��Ҫ�ɷ�ΪCuFeS2)ͨ���绯ѧ��Ӧת����ɣ��й�ת������ͼ��ת��ʱ�����ĵ缫��ӦʽΪ______________________________________________________��

���𰸡�2NA�� 1.204��1024 4Fe2++O2+4H+=4Fe3++2H2O ���ɵ�����Cu2S���棬�谭��ȡ Cu2S+2CuC12=4CuC1+S 2CuFeS2+6H++2e_=Cu2S+2Fe2++3H2S��
��������
��ͭ����S�ڸ��������գ�ʹ��ת��ΪFeS2��CuS������HCl��NaCl��CuCl2�����Һ ������ӦCu2++CuS+4Cl-=2[CuCl2]-+S�������˵õ���Һ��ͨ�����������Ӧ4CuCl2-+O2+4H+=4Cu2++8Cl-+2H2O��һ���¶��£������õ���Һ�м���ϡ���ᣬ������������ͭ���壬�ᾧ����õ�����ͭ���壬��������ԭ��Һ�õ�ͭ����������õ�FeS��S��FeS2ͨ��������յõ��������Ͷ����������������֣����������Ʊ����ᡣ
��1����ӦCu2S+4FeCl32CuCl2+4FeCl2+S����ͭ����ʧȥ��������ͭ���ӣ�������ʧȥ���������������ӵõ��������������ӣ�����2mol CuCl2����Ӧ��ת�Ƶ��ӵ���ĿΪ4mol��������1mol CuCl2����Ӧ��ת�Ƶ��ӵ���ĿΪ2mol��2NA���������������ױ�������������Ӧ��4Fe2++O2+4H+=4Fe3++2H2O���ʽ�ȡʱ�������������¿�ά��Fe3+�ϸ�Ũ�ȣ�
�ʴ�Ϊ��2NA�� 1.204��1024��4Fe2++O2+4H+=4Fe3++2H2O��
��2������ͼ��֪��δϴ��ϴȥ���ͭ�Ľ�ȡ�ʵͣ���Ϊ���ɵ�����Cu2S���棬�谭��ȡ���ʴ�Ϊ�����ɵ�����Cu2S���棬�谭��ȡ��
��3��353Kʱ����FeCl3��ȡҺ�м���CuCl2���Ȼ�ͭ������ͭ��ӦCu2S+2CuC12=4CuC1+S�����ɵ��Ȼ���ͭ���Ȼ�����ӦCuCl+FeCl3�TCuCl2+FeCl2���ܼӿ�ͭԪ�صĽ�ȡ���ʣ��ʴ�Ϊ��Cu2S+2CuC12=4CuC1+S��
��4�������õ��ӷ�����ԭ��Ӧ�����ϼ۽��ͣ�����ͼ��֪��CuFeS2�õ�������Cu2S��Fe2+��H2S���ʵ缫��ӦʽΪ��2CuFeS2+6H++2e-=Cu2S+2Fe2++3H2S�����ʴ�Ϊ��2CuFeS2+6H++2e-=Cu2S+2Fe2++3H2S����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ĸ���ͬ�������У��ڲ�ͬ����������N2+3H2==2NH3��Ӧ���ϳɰ���������������ͬʱ���ڲⶨ�Ľ���жϣ����ɰ������������� �� ��
A. v(H2)=0.1 mol��(L��min)1B. v(N2)=0.01 mol��(L��s)1
C. v(N2)=0.2 mol��(L��min)1D. v(NH3)=0.3 mol��(L��min)1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ч����SO2�Կ�������Ⱦ��
(1)��ú�м���ʯ��ʯ�ɼ���ȼ�ղ�����SO2�ĺ������÷�Ӧ�Ļ�ѧ����ʽ��
_______________________________��
(2)��ˮ�������ԣ���Ҫ����Na����K����Ca2����Mg2����Cl����SO42����Br����HCO3���ȡ���SO2�����������ú�ˮ�����乤��������ͼ��ʾ��

������������ͨ�������Ŀ����_____________________________________��
��ͨ��������������к�ˮ����Ȼ��ˮ��ȣ�Ũ�������Բ�ͬ��������________��
a��Cl�������� b��SO42�������� c��Br�������� d��HCO3��
(3)��NaOH��Һ���������е�SO2�������õ�Na2SO3��Һ���е�⣬�ɵõ�NaOH��ͬʱ�õ�H2SO4����ԭ����ͼ��ʾ(�缫����Ϊʯī)��

��ͼ��a��Ҫ���ӵ�Դ��________(����������������)����C��������������________��
��SO32���ŵ�ĵ缫��ӦʽΪ____________________________��
�۵�����������������������ǿ����ƽ���ƶ���ԭ������ԭ��
__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������У��뾶���ɴ�С����˳����ȷ����
�ٻ�̬X��ԭ�ӽṹʾ��ͼ
�ڻ�̬Y�ļ۵����Ų�ʽ��3s23p5
�ۻ�̬Z2���ĵ����Ų�ͼ![]()
��W��̬ԭ����2���ܲ㣬����ʽΪ![]()
A. ��>��>��>�� B. ��>��>��>��
C. ��>��>��>�� D. ��>��>��>��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Ϣ��ѧ����Դ��ѧ�е�һ����Ҫ���ϣ���������оƬ��̫���ܵ�صȡ������ǹ�ҵ����ȡ�����һ�ַ�����

��ش���������(��Ԫ������Ӧ��Ԫ�ط��ű�ʾ)��
(1)���������������У������û���Ӧ����____(�Ӧ����)��
(2)д����Ӧ�۵Ļ�ѧ����ʽ_______��
(3)������W����;�ܹ㣬ͨ��������������ҵ����ֽ��ҵ���ϼ�����������������������Ȼˮ������������ʯӢɰ�ʹ��һ��������ϼ�����1 373��1 623 K��Ӧ�����ɻ�����W���仯ѧ����ʽ��___��
(4)A��B��C���������������ܼ���������Ϊ����Ŀ���һ��������___(�ѧʽ)���ֱ�ͨ��W��Һ���ܵõ���ɫ������������______(�ѧʽ)��
(5)��ҵ�Ϻϳɰ���ԭ��H2���Ʒ����Ȱѽ�̿��ˮ������Ӧ����ˮú�������ᴿˮú���õ�������H2���ᴿˮú���õ�������H2�Ļ�ѧ����ʽΪ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ��������ʯ����̿��������ʯ��ʯΪԭ����ұ������
��1����д���ô������ڸ�����ұ�����Ļ�ѧ����ʽ___________�������û�ѧ�������ʵ�鷽������������Ӧ�õ��Ĺ�������к�������___________��
��2��С��ͬѧ����ұ�����õ����۰���ͼװ�����Ʊ� Fe(OH)2��ʵ�鿪ʼʱӦ��_______����a��___________���� b(����رա�)
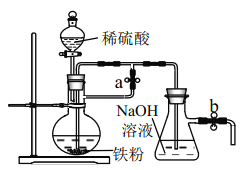
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F��G��H����Է�������������������壬���Ǿ��ɶ�����Ԫ����ɣ������������ʣ�
��B��ʹʪ��ĺ�ɫʯ����ֽ������A��C��D����ʹʪ���ʯ����ֽ��ɫ��E��G����ʹʪ�����ɫʯ����ֽ��죻
��F�ʺ���ɫ��
��G��H����ʹƷ����ɫ��A��H�а���ȼ�ղ�������ɫ���棻
��C��D����ȫȼ������E��H2O��ͬʱ�ų������ȣ���ҵ�Ͽ����ø÷�Ӧ���ӻ��и������
��ش��������⣺
(1)E�ĵ���ʽΪ_____��D������Ԫ�صĻ�̬ԭ�Ӻ�������Ų�ʽΪ___��C�����е������������ĸ���֮��Ϊ___��
(2)д��ʵ�����ù���ҩƷ��ȡB�Ļ�ѧ����ʽ_______________��
(3)����a��ͨ������G����b��ͨ������F��XΪ�Ȼ�����Һ���۲쵽��������_____________,
��Ӧ�����ӷ���ʽΪ_________________��

(4)��֪��E(g)+3A(g)![]() CH3OH(l)+H2O(l) ��H=-53.66 kJ��mol-1
CH3OH(l)+H2O(l) ��H=-53.66 kJ��mol-1
2CH3OH(l)![]() CH3OCH3(g)+H2O(l) ��H=-23.4 kJ��mol-1
CH3OCH3(g)+H2O(l) ��H=-23.4 kJ��mol-1
д��E�д���ʱ��A�ϳɶ�����(CH3OCH3)���Ȼ�ѧ����ʽ_____________��
(5)����C��ʹ�����ữ�ĸ��������Һ��ɫ������֮һ��E���÷�Ӧ�Ļ�ѧ����ʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧʽΪC5H7Cl���л����ṹ�������ǣ� ��
A.ֻ��1��![]() ����ֱ���л���
����ֱ���л���
B.��2��![]() ����ֱ���л���
����ֱ���л���
C.��1��![]() ���Ļ�״�л���
���Ļ�״�л���
D.��1����C![]() C������ֱ���л���
C������ֱ���л���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪ���־������ʾ��ͼ��


��ش��������⣺
��1�����������У�����֮���Թ��ۼ�����γɵľ�����____��
��2���������ʯ��MgO��CaCl2���ɱ�5�־�����۵��ɸߵ��͵�˳��Ϊ��___��
��3��NaCl������MgO������ͬ��NaCl����ľ�����___(����ڡ���С�ڡ�)MgO���壬ԭ����____��
��4��ÿ��Cu������ʵ��ռ��___��Cuԭ�ӣ�CaCl2������Ca2������λ��Ϊ__��
��5�������۵�Զ���ڸɱ�����H2O�Ǽ��Է��ӡ�CO2�ǷǼ��Է����⣬����һ����Ҫ��ԭ����_____��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com