
����Ŀ����ú��Ϊȼ�Ͽ�ͨ����������;����
;��I��C(s)+O2(g)=CO2(g) ��H1<0��
;��II�����Ƴ�ˮú����C(s)+H2O(g)=CO(g)+H2(g) ��H2>0��
��ȼ��ˮú����2CO(g)+O2(g)=2CO2(g) ��H3<0��
2H2(g)+O2(g)=2H2O(g) ��H4<0��
��ش���������:
��1��;��I�ų�������_____________( ����������������������С����) ;��II�ų���������
��2����H1����H2����H3����H4����ѧ��ϵʽ��_______________��
��3��12g ̿���������в���ȫȼ������һ����̼���ų�110.35kJ���������Ȼ�ѧ����ʽΪ_______________��
���𰸡����� ��H1=��H2+![]() (��H3+��H4) C(s) +
(��H3+��H4) C(s) +![]() O2(g) = CO(g) ��H=��110.35kJ/mol
O2(g) = CO(g) ��H=��110.35kJ/mol
��������
��1���ɸ�˹���ɿ�֪������һ����Ӧ���Էֲ����У��������Ӧ�����ջ�ų��������ܺ��������Ӧһ�η���ʱ���ջ�ų���������ͬ���ʴ�Ϊ�����ڣ�
��2�����ݸ�˹���ɣ���Ӧ1=��Ӧ2+��Ӧ3��![]() +��Ӧ4��
+��Ӧ4��![]() ��������H1=��H2+
��������H1=��H2+![]() (��H3+��H4)���ʴ�Ϊ����H1=��H2+
(��H3+��H4)���ʴ�Ϊ����H1=��H2+![]() (��H3+��H4)��
(��H3+��H4)��
��3��12g ̿���������в���ȫȼ������һ����̼���ų�110.35kJ��������1mol̿���������в���ȫȼ������һ����̼���ų�110.35kJ�������Ȼ�ѧ����ʽΪ��C(s)+![]() O2(g)=CO(g)��H=-110.35 kJmol-1���ʴ�Ϊ��C(s)+
O2(g)=CO(g)��H=-110.35 kJmol-1���ʴ�Ϊ��C(s)+![]() O2(g)=CO(g)��H=-110.35kJmol-1��
O2(g)=CO(g)��H=-110.35kJmol-1��


 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʱ������������ȷ����![]()
A.![]() ��
��![]() ��
��![]() �����Һ��
�����Һ��![]()
B.![]() �Ŀ�����
�Ŀ�����![]() ��
��![]() ������ˮ��
������ˮ��![]() ��10��
��10��
C.AgCl��![]()
![]() ��Һ��
��Һ��![]() NaCl��Һ�е��ܽ����ͬ
NaCl��Һ�е��ܽ����ͬ
D.![]() ��Һ��ˮϡ����100mL��pH��
��Һ��ˮϡ����100mL��pH��![]() ����С
����С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D�����л���ֱ���̼���⡢��Ԫ���е����ֻ�����Ԫ����ɸ�ȡ�����л���0.1mol���ֱ���ȫȼ�գ����ܵõ�4.48 L����״���£�������̼��D��ˮ��Һ�����ԡ������л���ת����ϵ��ͼ��
![]()
�ش��������⣺
��1��A��D���������������ŵ����Ʒֱ���______��______��
��2��a mol A��B�Ļ��������ȫȼ�գ��������������Ϊ______����״������
��3��B��C�Ļ�ѧ����ʽ________����Ӧ����______��
��4����ʵ�������������ͼ��ʾ��װ����ȡ����������
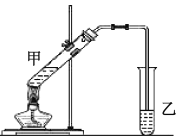
��д����![]() �����ᷢ��������Ӧ�Ļ�ѧ����ʽ_________��
�����ᷢ��������Ӧ�Ļ�ѧ����ʽ_________��
�ڷ�Ӧ��ʼǰ���Թ�����ʢ�ŵ��Լ�Ϊ______������������ʱ���Թܼ����Լ�����˳��Ϊ______��
�ۺϳ����������ķ�ӦΪ���ȷ�Ӧ��ʵ���������Ӧ�¶�Ӧ������85������Ϊ�ˡ���ʵ���¶Ȳ��˵���85�����ҵ�ԭ����________��
��5�����������������ֲ�Ʒ���ᴿ�������£���֪���Ȼ������Ҵ������������
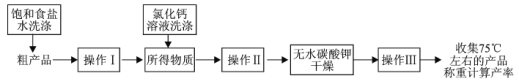
ͼ�в����������Ϊ_______�������������Ϊ_______�������������Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.2molij�л����0.5mol������һ�ܱ�������ȼ�յò���ΪCO2��CO��H2O(��������������ͨ��Ũ����ʱ��Ũ��������������� 10.8g����ͨ�����ȵ�����ͭʱ������ͭ������������ 3.2g����ͨ����ʯ��ʱ����ʯ�ҵ����������� 17.6g�����л���Ļ�ѧʽ��
A. C2H4 B. C2H6O C. C2H6O2 D. C3H6O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��ȷ���ǣ� ��
A.ǿ������Һ��NaClO��Fe(OH)3����ΪFeO42-��3ClO+2Fe(OH)3=2FeO42-+3Cl+4H++H2O
B.Ӳ֬�����Ҵ���������Ӧ��C17H35COOH+C2H518OH![]() C17H35COOC2H5+H218O
C17H35COOC2H5+H218O
C.��ϡ�����ȥ��������Һ����������������ƣ�S2O32-+2H+=SO2��+S��+H2O
D.��NH4HCO3��Һ�м�������ʯ��ˮ��Ca2++HCO3-+OH=CaCO3��+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ�����A��B��C��D�������ʻ�ϣ��������·�Ӧ��aA��bB![]() cC(s)��dD������Ӧ����һ��ʱ����A������nmol��B������
cC(s)��dD������Ӧ����һ��ʱ����A������nmol��B������![]() n mol��C������
n mol��C������![]() n mol��D������n mol����ʱ�ﵽ��ѧƽ�⡣����д���пհף�
n mol��D������n mol����ʱ�ﵽ��ѧƽ�⡣����д���пհף�
(1)�û�ѧ����ʽ�����ʵĻ�ѧ������Ϊa=_____��b=______��c=_____��d=______��
(2)��ֻ�ı�ѹǿ����Ӧ���ʷ����仯����ƽ�ⲻ�����ƶ����÷�Ӧ������D�ľۼ�״̬Ϊ______��
(3)��ֻ�����¶ȣ���Ӧһ��ʱ���֪�������������ʵ����ִﵽ��ȣ���÷�ӦΪ________��Ӧ(��������������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D�������ʶ������壬����5L���ܱ������н��з�Ӧ��4A��5B![]() 4C��6D��30s��C�����ʵ���������0.30mol���������й�������ȷ���ǣ� ��
4C��6D��30s��C�����ʵ���������0.30mol���������й�������ȷ���ǣ� ��
A.��Ӧ��ʼ��30s��v(A)��0.010mol��L��1��s��1
B.30sʱ������D�����ʵ�������Ϊ0.45mol
C.30sʱ������A��B��C��D�����ʵ���֮��һ����4��5��4��6
D.��Ӧ��ʼ��30s��������A�����ʵ���������0.30mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ظ����![]() �Ǹ��л�ѧ����������������ҵ���Ը�����Ϊԭ���ü����������Ʊ�����������ͨ������
�Ǹ��л�ѧ����������������ҵ���Ը�����Ϊԭ���ü����������Ʊ�����������ͨ������![]() ��FeO��
��FeO��![]() ��
��![]() �ȣ�
�ȣ�

��֪����![]() ��ˮǿ��ˮ�⣮
��ˮǿ��ˮ�⣮
��![]() ��ɫ
��ɫ![]() ��ɫ
��ɫ![]()
��ش��������⣺
(1)����ʯ�����Ŀ���� ______ ����������ʱ![]() ������Ӧ�Ļ�ѧ����ʽΪ ______ ��
������Ӧ�Ļ�ѧ����ʽΪ ______ ��
(2)����1���к��ɫ���ʣ�д�����ɸ����ʷ�Ӧ�����ӷ���ʽ ______ ![]() ����2����Ҫ�ɷ���
����2����Ҫ�ɷ���![]() �� ______ ��
�� ______ ��
(3)�ü�Ҫ������˵��![]() ��Һ�м���KCl���壬��������
��Һ�м���KCl���壬��������![]() ��ԭ�� ______ ��
��ԭ�� ______ ��
(4)![]() ʱ���Է�Ӧ
ʱ���Է�Ӧ![]() ��ɫ
��ɫ![]() ��ɫ
��ɫ![]() ��ȡ
��ȡ![]() ��Һ����ʵ�飬��ò���ʵ���������£�
��Һ����ʵ�飬��ò���ʵ���������£�
ʱ�� | 0 |
|
|
|
|
|
|
|
|
| |
| 0 |
|
|
|
�ٷ�Ӧ�ﵽƽ��ʱ����Һ��![]() ���÷�Ӧƽ�ⳣ��KΪ ______ ��
���÷�Ӧƽ�ⳣ��KΪ ______ ��
�������й�˵����ȷ�� ______ ��
![]() ������
������![]() ���壬��ʹ��Һ�ij�ɫ����
���壬��ʹ��Һ�ij�ɫ����
![]() ʱ
ʱ![]()
![]() ��Һ��
��Һ��![]() ��
��![]() ��1ʱ�÷�Ӧ�Ѵ�ƽ��״̬
��1ʱ�÷�Ӧ�Ѵ�ƽ��״̬
![]() ��Ӧ�ﵽƽ��ʱ
��Ӧ�ﵽƽ��ʱ![]() ��ת����Ϊ
��ת����Ϊ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ת���У�A��һ�����Σ�D����Է���������C����Է���������16��E���ᣬ��X������ǿ�ỹ��ǿ��ʱ���������µ�ת����ϵ��
A![]() B
B![]() C
C![]() D
D![]() E
E
��X��ǿ��ʱ��A��B��C��D��E����ͬһ��Ԫ�أ���X��ǿ��ʱ��A��B��C��D��E��������ͬһ��Ԫ�ء���ش�
(1)A��________��Y��________��Z��________��
(2)��X��ǿ��ʱ��E��________��д��B����C�Ļ�ѧ����ʽ��________________��
(3)��X��ǿ��ʱ��E��________��д��B����C�Ļ�ѧ����ʽ��_________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com