
Α―ΟΚΉςΈΣ»ΦΝœΩ…Ά®Ιΐœ¬Ν–ΝΫ÷÷ΆΨΨΕ:
ΆΨΨΕΔώΓΓC(s)+O2(g) CO2(g)ΓΓΠΛH1<0ΔΌ
CO2(g)ΓΓΠΛH1<0ΔΌ
ΆΨΨΕΔρΓΓœ»÷Τ≥…Υ°ΟΚΤχ:
C(s)+H2O(g) CO(g)+H2(g)ΓΓΠΛH2>0ΔΎ
CO(g)+H2(g)ΓΓΠΛH2>0ΔΎ
‘Ό»Φ…’Υ°ΟΚΤχ:
2CO(g)+O2(g) 2CO2(g)ΓΓΠΛH3<0Δέ
2CO2(g)ΓΓΠΛH3<0Δέ
2H2(g)+O2(g) 2H2O(g)ΓΓΠΛH4<0Δή
2H2O(g)ΓΓΠΛH4<0Δή
«κΜΊ¥πœ¬Ν–Έ Χβ:
(1)ΆΨΨΕΔώΖ≈≥ωΒΡ»»ΝΩάμ¬έ…œΓΓΓΓΓΓΓΓ(ΧνΓΑ¥σ”ΎΓ±ΓΑΒ»”ΎΓ±ΜρΓΑ–Γ”ΎΓ±)ΆΨΨΕΔρΖ≈≥ωΒΡ»»ΝΩΓΘ
(2)ΠΛH1ΓΔΠΛH2ΓΔΠΛH3ΓΔΠΛH4ΒΡ ΐ―ßΙΊœΒ Ϋ «ΓΓΓΘ
(3)“―÷Σ:ΔΌC(s)+O2(g) CO2(g)ΓΓΠΛH1=-393.5 kJΓΛmol-1
CO2(g)ΓΓΠΛH1=-393.5 kJΓΛmol-1
ΔΎ2CO(g)+O2(g) 2CO2(g)ΓΓΠΛH2=-566 kJΓΛmol-1
2CO2(g)ΓΓΠΛH2=-566 kJΓΛmol-1
ΔέTiO2(s)+2Cl2(g) TiCl4(s)+O2(g)ΓΓΠΛH3=+141 kJΓΛmol-1
TiCl4(s)+O2(g)ΓΓΠΛH3=+141 kJΓΛmol-1
‘ρTiO2(s)+2Cl2(g)+2C(s) TiCl4(s)+2CO(g)ΒΡΠΛH=ΓΓΓΓΓΓΓΓΓΘ
TiCl4(s)+2CO(g)ΒΡΠΛH=ΓΓΓΓΓΓΓΓΓΘ
(4)“―÷Σœ¬Ν–ΗςΉι»»Μ·―ßΖΫ≥Χ Ϋ
ΔΌFe2O3(s)+3CO(g) 2Fe(s)+3CO2(g)ΓΓΠΛH1=-25 kJΓΛmol-1
2Fe(s)+3CO2(g)ΓΓΠΛH1=-25 kJΓΛmol-1
ΔΎ3Fe2O3(s)+CO(g) 2Fe3O4(s)+CO2(g)ΓΓΠΛH2=-47 kJΓΛmol-1
2Fe3O4(s)+CO2(g)ΓΓΠΛH2=-47 kJΓΛmol-1
ΔέFe3O4(s)+CO(g) 3FeO(s)+CO2(g)ΓΓΠΛH3=+640 kJΓΛmol-1
3FeO(s)+CO2(g)ΓΓΠΛH3=+640 kJΓΛmol-1
«κ–¥≥ωFeO(s)±ΜCO(g)ΜΙ‘≠≥…FeΚΆCO2(g)ΒΡ»»Μ·―ßΖΫ≥Χ ΫΓΓ______________________ΓΘ
(1)Β»”ΎΓΓ
(2)ΠΛH1=ΠΛH2+(ΠΛH3+ΠΛH4)/2
(3)-80 kJΓΛmol-1
(4)FeO(s)+CO(g) Fe(s)+CO2(g)ΓΓΠΛH=-218 kJΓΛmol-1
Fe(s)+CO2(g)ΓΓΠΛH=-218 kJΓΛmol-1
ΓΨΫβΈωΓΩ(1)ΗυΨίΗ«ΥΙΕ®¬…ΒΡΚ§“ε÷Σ,ΆΨΨΕΔώΖ≈≥ωΒΡ»»ΝΩΒ»”ΎΆΨΨΕΔρΖ≈≥ωΒΡ»»ΝΩΓΘ
(2)”…Η«ΥΙΕ®¬…:ΔΌ=ΒΟΠΛH1=ΠΛH2+ΠΛH3+ΠΛH4ΓΘ
(3)”…Δέ+2ΓΝΔΌ-ΔΎΒΟΥυ«σΖΫ≥Χ Ϋ,Φ¥ΠΛH=ΠΛH3+2ΠΛH1-ΠΛH2=+141 kJΓΛmol-1+2ΓΝ(-393.5 kJΓΛmol-1)-(-566 kJΓΛmol-1)=-80 kJΓΛmol-1ΓΘ
(4)”…Χβ“β:ΔήFeO(s)+CO(g) Fe(s)+CO2(g)ΓΓΠΛH
Fe(s)+CO2(g)ΓΓΠΛH
‘ρΔή=ΒΟ ΠΛH==-218 kJΓΛmol-1
‘ρFeO(s)+CO(g) Fe(s)+CO2(g)ΓΓΠΛH=-218 kJΓΛmol-1
Fe(s)+CO2(g)ΓΓΠΛH=-218 kJΓΛmol-1


 ‘ΤΡœ Π¥σΗΫ–Γ“ΜœΏΟϊ ΠΧα”≈Ής“ΒœΒΝ–¥πΑΗ
‘ΤΡœ Π¥σΗΫ–Γ“ΜœΏΟϊ ΠΧα”≈Ής“ΒœΒΝ–¥πΑΗ ≥ε¥Χ100Ζ÷ΒΞ‘Σ”≈Μ·ΝΖΩΦΨμœΒΝ–¥πΑΗ
≥ε¥Χ100Ζ÷ΒΞ‘Σ”≈Μ·ΝΖΩΦΨμœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΕΰΜ·―ßΥ’ΫΧΑφ―Γ–ό2 1.3ΚΘΥ°Β≠Μ·ΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
ΒΊ«ρΒΡΚΘΥ°ΉήΝΩ‘Φ”–1.4ΓΝ1018 tΘ§ «»ΥάύΉν¥σΒΡΉ ‘¥Ωβ
Θ®1Θ©»γΆΦάϊ”ΟΚΘΥ°ΒΟΒΫΒ≠Υ°ΒΡΖΫΖ®ΈΣ________ΓΘ

Θ®2Θ©Βγ…χΈωΖ® «ΫϋΡξΖΔ’ΙΤπά¥ΒΡ“Μ÷÷ΫœΚΟΒΡΚΘΥ°Β≠Μ·ΦΦ θΘ§Τδ‘≠άμ»γΆΦΘΚ

a «Βγ‘¥ΒΡ________ΦΪΘΜIΩΎ≈≈≥ωΒΡ «________Θ®ΧνΓΑΒ≠Υ°Γ±ΜρΓΑ≈®Υ°Γ±Θ©ΓΘ
Θ®3Θ©ΚΘΥ°Β≠Μ·ΚσΒΡ≈®Υ°÷–Κ§¥σΝΩ―ΈΖ÷Θ§≈≈»κΥ°÷–ΜαΗΡ±δΥ°÷ Θ§≈≈ΒΫΆΝ»ά÷–ΜαΒΦ÷¬ΆΝ»ά―ΈΦνΜ·Θ§Ι ≤ΜΡή÷±Ϋ”≈≈Ζ≈Θ§Ω…“‘”ꬻΦνΙΛ“ΒΝΣ≤ζΓΘ
ΔΌΒγΫβ«Α–η“ΣΑ―≈®Υ°ΨΪ÷ΤΘ§Φ”»κ ‘ΦΝΒΡΥ≥–ρΈΣ___________________________________ΘΜ
ΔΎ”ΟάκΉ”ΫΜΜΜΡΛΒγΫβ≤έΒγΫβ ≥―ΈΥ°ΒΡΜ·―ßΖΫ≥Χ ΫΈΣ
_________________________________________________________________________________________________________________________________ΓΘ
Θ®4Θ©”Υ «ΚΥΖ¥”ΠΉν÷Ί“ΣΒΡ»ΦΝœΘ§ΤδΧαΝΕΦΦ θ÷±Ϋ”ΙΊœΒΉ≈“ΜΗωΙζΦ“ΚΥΙΛ“ΒΜρΚΥΈδΤςΒΡΖΔ’ΙΥ°ΤΫΘ§ΚΘΥ°÷–”Υ“‘UCl4–Έ Ϋ¥φ‘ΎΘ§ΟΩΕ÷ΚΘΥ°÷ΜΚ§3.3 mg”ΥΘ§ΚΘΥ°ΉήΝΩΦΪ¥σΘ§”ΥΉήΝΩœύΒ±Ψό¥σΓΘ≤Μ…ΌΙζΦ“’ΐ‘ΎΧΫΥςΚΘΥ°Χα”ΥΒΡΖΫΖ®ΓΘœ÷‘ΎΘ§“―Ψ≠―–÷Τ≥…ΙΠΝΥ“Μ÷÷ρϋΚœ–ΆάκΉ”ΫΜΜΜ ς÷§Θ§ΥϋΉ®Ο≈ΈϋΗΫΚΘΥ°÷–ΒΡ”ΥΘ§Εχ≤ΜΈϋΗΫΤδΥϊ‘ΣΥΊΓΘΤδΖ¥”Π‘≠άμΈΣ________Θ® ς÷§”ΟHR¥ζΧφΘ©Θ§ΖΔ…ζάκΉ”ΫΜΜΜΚσΒΡάκΉ”ΫΜΜΜΡΛ”ΟΥα¥ΠάμΜΙΩ…‘Ό…ζ≤ΔΒΟΒΫΚ§”ΥΒΡ»ή“ΚΘ§ΤδΖ¥”Π‘≠άμΈΣ_______________________________________________________________________________________________________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΕΰΜ·―ß»ΥΫΧΑφ―Γ–όΥΡ 7Μ·―ßΤΫΚβΉ¥Χ§ΒΡΫ®ΝΔΦΑ±ξ÷ΨΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
‘ΎΚψΈ¬ΓΔΚψ»ίœ¬,Β±Ζ¥”Π»ίΤςΡΎΉή―Ι«Ω≤ΜΥφ ±Φδ±δΜ· ±,œ¬Ν–Ω…ΡφΖ¥”Π“ΜΕ®¥οΒΫΤΫΚβΒΡ «(ΓΓΓΓ)
A.A(g)+B(g) C(g)B.A(g)+2B(g)
C(g)B.A(g)+2B(g) 3C(g)
3C(g)
C.A(g)+B(g) C(g)+D(g)D.“‘…œΕΦ¥οΒΫΤΫΚβ
C(g)+D(g)D.“‘…œΕΦ¥οΒΫΤΫΚβ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΕΰΜ·―ß»ΥΫΧΑφ―Γ–όΥΡ 5Μ·―ßΖ¥”ΠΥΌ¬ ΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
‘Ύ2 LΟή±’»ίΤς÷–Ϋχ––Ζ¥”Π:mX(g)+nY(g) pZ(g)+qQ(g), Ϋ÷–mΓΔnΓΔpΓΔqΈΣΜ·―ßΦΤΝΩ ΐΓΘ‘Ύ0~3 minΡΎ,ΗςΈο÷ Έο÷ ΒΡΝΩΒΡ±δΜ·»γœ¬±μΥυ Ψ:
pZ(g)+qQ(g), Ϋ÷–mΓΔnΓΔpΓΔqΈΣΜ·―ßΦΤΝΩ ΐΓΘ‘Ύ0~3 minΡΎ,ΗςΈο÷ Έο÷ ΒΡΝΩΒΡ±δΜ·»γœ¬±μΥυ Ψ:
ΓΓ Έο÷ ±ΦδΓΓ | X | Y | Z | Q |
Τπ Φ/mol | 0.7 |
| 1 |
|
2 minΡ©/mol | 0.8 | 2.7 | 0.8 | 2.7 |
3 minΡ©/mol |
|
| 0.8 |
|
“―÷Σ2 minΡΎv(Q)=0.075 molΓΛL-1ΓΛmin-1,,
(1) ‘»ΖΕ®“‘œ¬Έο÷ ΒΡœύΙΊΝΩ:
Τπ Φ ±n(Y)=ΓΓΓΓΓΓΓΓ,n(Q)=ΓΓΓΓΓΓΓΓΓΘ
(2)ΖΫ≥Χ Ϋ÷–m=ΓΓΓΓΓΓΓΓ,n=ΓΓΓΓΓΓΓΓ,p=ΓΓΓΓΓΓΓΓ,q=ΓΓΓΓΓΓΓΓΓΘ
(3)”ΟZ±μ Ψ2 minΡΎΒΡΖ¥”ΠΥΌ¬ ΓΓΓΓΓΓΓΓΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΕΰΜ·―ß»ΥΫΧΑφ―Γ–όΥΡ 5Μ·―ßΖ¥”ΠΥΌ¬ ΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
Ζ¥”Π4A(s)+3B(g)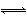 2C(g)+D(g),Ψ≠2 min BΒΡ≈®Ε»Φθ…Ό0.6 molΓΛL-1ΓΘΕ‘¥ΥΖ¥”ΠΥΌ¬ ΒΡ’ΐ»Ζ±μ Ψ «(ΓΓΓΓ)
2C(g)+D(g),Ψ≠2 min BΒΡ≈®Ε»Φθ…Ό0.6 molΓΛL-1ΓΘΕ‘¥ΥΖ¥”ΠΥΌ¬ ΒΡ’ΐ»Ζ±μ Ψ «(ΓΓΓΓ)
A.”ΟA±μ ΨΒΡΖ¥”ΠΥΌ¬ «0.8 molΓΛL-1ΓΛs-1
B.Ζ÷±π”ΟBΓΔCΓΔD±μ ΨΖ¥”ΠΒΡΥΌ¬ ,Τδ±»÷Β «3ΓΟ2ΓΟ1
C.‘Ύ2 minΡ© ±ΒΡΖ¥”ΠΥΌ¬ ,”ΟΖ¥”ΠΈοBά¥±μ Ψ «0.3 molΓΛL-1ΓΛmin-1
D.‘Ύ’β2 minΡΎ”ΟBΚΆC±μ ΨΒΡΖ¥”ΠΥΌ¬ ΒΡ÷ΒΕΦ «œύΆ§ΒΡ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΕΰΜ·―ß»ΥΫΧΑφ―Γ–όΥΡ 4Μ·―ßΖ¥”Π»»ΒΡΦΤΥψΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
“―÷Σœ¬Ν–Ζ¥”ΠΒΡΖ¥”Π»»:
(1)CH3COOH(l)+2O2(g) 2CO2(g)+2H2O(l)ΓΓΠΛH1=-870.3 kJΓΛmol-1
2CO2(g)+2H2O(l)ΓΓΠΛH1=-870.3 kJΓΛmol-1
(2)C(s)+O2(g) CO2(g)ΓΓΠΛH2=-393.5 kJΓΛmol-1
CO2(g)ΓΓΠΛH2=-393.5 kJΓΛmol-1
(3)H2(g)+O2(g) H2O(l)ΓΓΠΛH3=-285.8 kJΓΛmol-1
H2O(l)ΓΓΠΛH3=-285.8 kJΓΛmol-1
‘ρœ¬Ν–Ζ¥”ΠΒΡΖ¥”Π»»ΈΣ(ΓΓΓΓ)
2C(s)+2H2(g)+O2(g) CH3COOH(l)
CH3COOH(l)
A.ΠΛH=+488.3 kJΓΛmol-1B.ΠΛH=-244.15 kJΓΛmol-1
C.ΠΛH=-977.6 kJΓΛmol-1D.ΠΛH=-488.3 kJΓΛmol-1
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΕΰΜ·―ß»ΥΫΧΑφ―Γ–όΥΡ 3»Φ…’»» Ρή‘¥ΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
“―÷Σ:
ΔΌ25 ΓφΓΔ101 kPa ±,2C(s)+O2(g) 2CO(g)ΓΓΠΛH=-221 kJΓΛmol-1
2CO(g)ΓΓΠΛH=-221 kJΓΛmol-1
ΔΎœΓ»ή“Κ÷–,H+(aq)+OH-(aq) H2O(l)ΓΓΠΛH=-57.3 kJΓΛmol-1
H2O(l)ΓΓΠΛH=-57.3 kJΓΛmol-1
”÷“―÷Σ»θΒγΫβ÷ ΒγάκΈϋ»»ΓΘœ¬Ν–Ϋα¬έ’ΐ»ΖΒΡ «(ΓΓΓΓ)
A.CΒΡ»Φ…’»»¥σ”Ύ110.5 kJΓΛmol-1
B.ΔΌΒΡΖ¥”Π»»ΈΣ221 kJΓΛmol-1
C.œΓΝρΥα”κœΓNaOH»ή“ΚΖ¥”ΠΒΡ÷–ΚΆ»»ΈΣ-57.3 kJΓΛmol-1
D.œΓ¥ΉΥα”κœΓNaOH»ή“ΚΖ¥”Π…ζ≥…1 mol Υ°,Ζ≈≥ω57.3 kJ»»ΝΩ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΕΰΜ·―ß»ΥΫΧΑφ―Γ–όΥΡ 22ΒγΫβ‘≠άμΒΡ”Π”ΟΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΦΤΥψΧβ
œρ8 gΡ≥ΕΰΦέΫπ τΒΡ―θΜ·ΈοΙΧΧε÷–Φ”»κœΓΝρΥα, ΙΤδ«ΓΚΟΆξ»Ϊ»ήΫβ,“―÷ΣœϊΚΡΝρΥαΒΡΧεΜΐΈΣ100 mL,‘ΎΥυΒΟ»ή“Κ÷–≤ε»κ≤§ΒγΦΪΫχ––ΒγΫβ,Ά®Βγ“ΜΕ® ±ΦδΚσ,‘Ύ“ΜΗωΒγΦΪ…œ ’Φ·ΒΫ224 mL(±ξΉΦΉ¥Ωω)―θΤχ,‘ΎΝμ“ΜΗωΒγΦΪ…œΈω≥ωΗΟΫπ τ1.28 gΓΘ
(1)ΗυΨίΦΤΥψ»ΖΕ®Ϋπ τ―θΜ·ΈοΒΡΟϊ≥ΤΓΘ
(2)«σΆ®ΒγΚσΝρΥα»ή“ΚΒΡΈο÷ ΒΡΝΩ≈®Ε»(»ή“ΚΧεΜΐΑ¥100 mLΦΤΥψ)ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΡξΗΏΕΰΜ·―ß»ΥΫΧΑφ―Γ–όΥΡ 19“Μ¥ΈΒγ≥Ί Εΰ¥ΈΒγ≥ΊΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
≈ΠΩέΒγ≥ΊΩ…”Ο”ΎΦΤΥψΤςΓΔΒγΉ”±μΒ»ΒΡΒγ‘¥ΓΘ”–“Μ÷÷≈ΠΩέΒγ≥Ί,ΤδΒγΦΪΖ÷±πΈΣZnΚΆAg2O,“‘KOH»ή“ΚΈΣΒγΫβ÷ »ή“Κ,Βγ≥ΊΒΡΉήΖ¥”ΠΈΣZn+Ag2O+H2O 2Ag+Zn(OH)2ΓΘΙΊ”ΎΗΟΒγ≥ΊΒΡ–π ω≤Μ’ΐ»ΖΒΡ «(ΓΓΓΓ)
2Ag+Zn(OH)2ΓΘΙΊ”ΎΗΟΒγ≥ΊΒΡ–π ω≤Μ’ΐ»ΖΒΡ «(ΓΓΓΓ)

A. Ι”Ο ±ΒγΉ””…ZnΦΪΨ≠ΆβΒγ¬ΖΝςœρAg2OΦΪ,Zn «ΗΚΦΪ
B. Ι”Ο ±ΒγΉ””…Ag2OΦΪΨ≠ΆβΒγ¬ΖΝςœρZnΦΪ,Ag2O «ΗΚΦΪ
C.’ΐΦΪΒΡΒγΦΪΖ¥”ΠΈΣAg2O+2e-+H2O 2Ag+2OH-
2Ag+2OH-
D.ZnΦΪΖΔ…ζ―θΜ·Ζ¥”Π,Ag2OΦΪΖΔ…ζΜΙ‘≠Ζ¥”Π
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com