
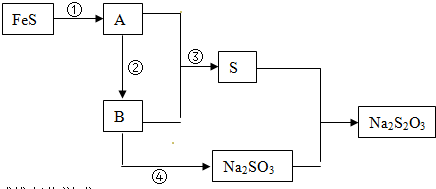
���� ��һ����ĩ���ܺ���K+��Fe3+��Al3+��Cl-��SO42-��CO32-�е������֣�������ʵ�飺
��ȡ�������壬����ϡ������裬����ȫ���ܽ⣬û������ų�������Һ��һ������CO32-��
�������Һ�м���һ����Ba��OH��2��Һ��������ɫ����������Һ�к���Fe3+��Al3+��SO42-�����߶��У����˺�����Һ�е���AgNO3��Һ���а�ɫ�������ɣ�����Һ�к���Cl-��
��ȡ���е���ɫ��������������ϡ�������ȫ���ܽ⣬�����ᱵ���������ᣬ����Һ��һ������SO42-��
������ȡ�������������������ˮ�������ȫ���ܽ⣬�õ�������Һ��
����ܵ���Һ�м��백ˮʹ��Һ�ʼ��ԣ��г������ɣ����ˣ����õ��ij����м��������NaOH��Һ���������٣���������������ǿ������������ܣ���˵����Һ�к���Fe3+��Al3+��
ͨ�����Ϸ���֪������ȷ����Һ���Ƿ���K+���Դ������
��� �⣺��һ����ĩ���ܺ���K+��Fe3+��Al3+��Cl-��SO42-��CO32-�е������֣�������ʵ�飺
��ȡ�������壬����ϡ������裬����ȫ���ܽ⣬û������ų�������Һ��һ������CO32-��
�������Һ�м���һ����Ba��OH��2��Һ��������ɫ����������Һ�к���Fe3+��Al3+��SO42-�����߶��У����˺�����Һ�е���AgNO3��Һ���а�ɫ�������ɣ�����Һ�к���Cl-��
��ȡ���е���ɫ��������������ϡ�������ȫ���ܽ⣬�����ᱵ���������ᣬ����Һ��һ������SO42-��
������ȡ�������������������ˮ�������ȫ���ܽ⣬�õ�������Һ��
����ܵ���Һ�м��백ˮʹ��Һ�ʼ��ԣ��г������ɣ����ˣ����õ��ij����м��������NaOH��Һ���������٣���������������ǿ������������ܣ���˵����Һ�к���Fe3+��Al3+��
ͨ�����Ϸ���֪������ȷ����Һ���Ƿ���K+��
��1������������֪������Һ��һ��������������CO32-��SO42-��һ�����е�������Fe3+��Al3+��Cl-������ȷ���Ƿ��е�������K+��Ҫ����KԪ����Ҫͨ����ɫ��Ӧ���ʴ�Ϊ��Fe3+��Al3+��Cl-����ɫ��Ӧ��
��2���������ɳ�����Ӧ���й����ӷ���ʽΪFe3++3NH3•H2O=Fe��OH��3��+3NH4+��Al3++3NH3•H2O=Al��OH��3��+3NH4+��
�ʴ�Ϊ��Fe3++3NH3•H2O=Fe��OH��3��+3NH4+��Al3++3NH3•H2O=Al��OH��3��+3NH4+��
��3�����������������������Ʒ�Ӧ���ɿ�����ƫ�����ƶ����³������٣���ѧ����ʽΪAl��OH��3+NaOH=NaAlO2+2H2O���ʴ�Ϊ��Al��OH��3+NaOH=NaAlO2+2H2O��
���� ���⿼�������ӷ�Ӧ�������ӵ��ƶϣ�Ϊ��Ƶ���㣬�������ɵij�������������ɫ�����ӹ���ͷ�Ӧȥȷ�����ڵ�����Ϊ���Ĺؼ���ע�����ճ������ӵ����ʼ����鷽������Ŀ�Ѷ��еȣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 20.7% | B�� | 22% | C�� | 1.3% | D�� | 7.3% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 18gNH4+�������ӵ���ĿΪ10NA | |
| B�� | 1molNa2O2������CO2��Ӧʱ��ת�Ƶ��ӵ���ĿΪ2NA | |
| C�� | ��״���£������Ϊ22.4L��O2��HCl��H2O���еķ�����Ŀ��ΪNA | |
| D�� | ����Ϊ16g��O2��O3�Ļ��������������ԭ�ӵ���ĿΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ�ʻ���ɫ���Ҿ��д̼�����ζ��˵��Cl2�Ĵ��� | |
| B�� | ������ɫ��������ɫ������ɫ��˵����Һ����Cl2���� | |
| C�� | ����NaHCO3��Һ������ɫ���������˵����HClO���� | |
| D�� | �ȼ��������ữ���ټ�AgNO3��Һ������ɫ������˵����Cl-���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����˷�Ӧ�Ļ�� | B�� | �����˷�Ӧ������������ | ||
| C�� | �����˷�Ӧ���ʱ� | D�� | �����˷�Ӧ��ƽ�ⳣ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڵ������Һ��ע��һ�������������ʹ��Һ�ָ���ԭʼ״̬ | |
| B�� | �ڵ������Һ��ͨ������4.48L���Ȼ������岢ע��0.1mol��ˮ����ʹ��Һ�ָ���ԭʼ״̬ | |
| C�� | ������������������ʵ���������������������ʵ�����0.75�� | |
| D�� | ���������������ƽ��Ħ������Ϊ58g/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ��Һ�п϶�����Fe2+��NO3-��SiO32-��I- | |
| B�� | ԭ��Һ�п϶�����K+��Fe3+��Fe2+��NO3-��SO42- | |
| C�� | ���������ɫ������NO���壬��CO2������� | |
| D�� | Ϊȷ���Ƿ���Cl-��ȡԭ��Һ���������������Һ���۲��Ƿ������ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
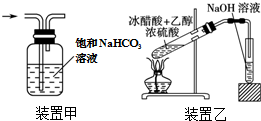
| A�� | ��25.0gCuSO4•5H2O����100mL����ˮ�����1.0mol•L-1����ͭ��Һ | |
| B�� | ��ɫ��Ӧʵ���У���պȡ������Һǰ������ϡ����ϴ����˿������������Ϊ��ɫ | |
| C�� | ��װ�ü׳�ȥCl2�е�HCl���� | |
| D�� | ��װ������ȡ�������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com