
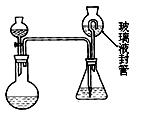
 ij����ѧϰС���ͬѧ��֤ʵ���е���Ч�ɷֲ��ⶨ�������ȵĺ���������Ʒ���ᷴӦ�����������ȵ���������Ʒ�����ı�ֵ������������ش��������⣺
ij����ѧϰС���ͬѧ��֤ʵ���е���Ч�ɷֲ��ⶨ�������ȵĺ���������Ʒ���ᷴӦ�����������ȵ���������Ʒ�����ı�ֵ������������ش��������⣺ ��1��Ϊ֤ʵ���ȶ��Զ���������Һ���к��������ӣ�����ȷ�IJ��������ǣ� ��
��1��Ϊ֤ʵ���ȶ��Զ���������Һ���к��������ӣ�����ȷ�IJ��������ǣ� �� ��2��Ϊ�ⶨ���ȶ��Զ���������Һ���ж������ȵĺ������ֽ������²�������ȡmg��2g���ң�
��2��Ϊ�ⶨ���ȶ��Զ���������Һ���ж������ȵĺ������ֽ������²�������ȡmg��2g���ң� ������������ƿ�У����Һ©���м���10mL������Һ��������ƿ�м���4g�⻯�أ���100mLˮ�ܽ���ټ�3mL������Һ�����ڲ���Һ����м���ˮ���ܽ���Һ©���е�������Һ������ƿ�У��ر�����������������ƿ��ʹ�����Ķ�����������ȫ��ͨ����������ƿ�б����գ��ݽ�����Һ����е�ˮ��Һ������ƿ�У����뼸�ε�����Һ����cmol/L��������Ʊ���Һ�ζ�����ɫ��ʧ(I2+2S2O32-=2I- +S4O62-)������ȥVmL�����������Һ��
������������ƿ�У����Һ©���м���10mL������Һ��������ƿ�м���4g�⻯�أ���100mLˮ�ܽ���ټ�3mL������Һ�����ڲ���Һ����м���ˮ���ܽ���Һ©���е�������Һ������ƿ�У��ر�����������������ƿ��ʹ�����Ķ�����������ȫ��ͨ����������ƿ�б����գ��ݽ�����Һ����е�ˮ��Һ������ƿ�У����뼸�ε�����Һ����cmol/L��������Ʊ���Һ�ζ�����ɫ��ʧ(I2+2S2O32-=2I- +S4O62-)������ȥVmL�����������Һ�� ����ʽΪ��
����ʽΪ�� �������ȱ���ԭΪ�����ӣ��÷�Ӧ�����ӷ���ʽΪ�� ��
�������ȱ���ԭΪ�����ӣ��÷�Ӧ�����ӷ���ʽΪ�� �� ����װ���в���Һ��ܵ������ǣ� �����ȷ����ƿ��
����װ���в���Һ��ܵ������ǣ� �����ȷ����ƿ�� �Ķ�������ȫ������ƿ�е�Һ������ ��
�Ķ�������ȫ������ƿ�е�Һ������ �� �����ȶ��Զ���������Һ���У�ClO2����������Ϊ
�����ȶ��Զ���������Һ���У�ClO2����������Ϊ  ����m��c��V��ʾ����
����m��c��V��ʾ����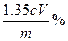 . ��2�֣�
. ��2�֣�

 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
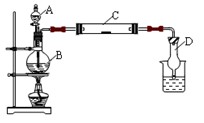
| A��ʵ�������õ��ĵζ��ܡ�����ƿ����ʹ��ǰ����Ҫ��©�� |
| B�����ʵ��������60mL ��ϡ�������Һ������ʱӦѡ��100mL����ƿ�� |
| C������ƿ�к�����������ˮ���ᵼ���������Һ��Ũ��ƫС�� |
| D����ʽ�ζ���������ˮϴ�Ӻ�װ���Ũ�ȵ�ϡ���ᣬ���õ�NaOH��Һ��Ũ�Ƚ�ƫ�� |
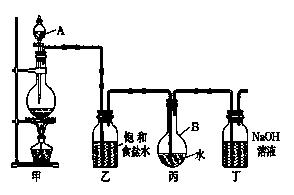
 �����ű�ʾ ����
�����ű�ʾ ���� _______________________________________________________________________
_______________________________________________________________________�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��һ�ִ�����Ⱦ�ij��ȤС����̽��
��һ�ִ�����Ⱦ�ij��ȤС����̽�� �����ʼ���ɫʵ��ķ�����������·�����
�����ʼ���ɫʵ��ķ�����������·�����


 I����֤
I����֤ �������ԣ�Cװ���п�ѡ�Լ� (�����)��
�������ԣ�Cװ���п�ѡ�Լ� (�����)��| A��Ba(HCO3)2��Һ | B�������� | C����ˮ | D��Ʒ����Һ |
 �Ļ�ԭ�ԣ�Cװ���п����Լ� (������)��
�Ļ�ԭ�ԣ�Cװ���п����Լ� (������)�� �Ļ�ѧ��Ӧ����ʽΪ��___________________________________________________��
�Ļ�ѧ��Ӧ����ʽΪ��___________________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������




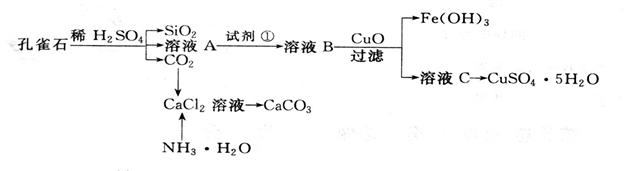

 ��ش��������⣺
��ش��������⣺ ��1����ҺA�Ľ���������__________��������ҺA��Fe3+������Լ�Ϊ ��
��1����ҺA�Ľ���������__________��������ҺA��Fe3+������Լ�Ϊ ��  A��KMnO4�� ���� B��(NH4) 2S C��H2O2 D��KSCN
A��KMnO4�� ���� B��(NH4) 2S C��H2O2 D��KSCN  ��2������ҺC���CuSO4��5H2O����Ҫ������������������ ���������˵Ȳ�����
��2������ҺC���CuSO4��5H2O����Ҫ������������������ ���������˵Ȳ����� ��3���Ʊ�CaCO3ʱ��Ӧ��CaCl2��Һ���ȼ���NH3.H2O,Ȼ��ͨ��CO2����Ӧ�ķ���ʽ��__________________________________________________________________��
��3���Ʊ�CaCO3ʱ��Ӧ��CaCl2��Һ���ȼ���NH3.H2O,Ȼ��ͨ��CO2����Ӧ�ķ���ʽ��__________________________________________________________________��


 ��4�����ⶨ��ҺA��Fe2+��Ũ�ȣ���Ҫ������ƿ����ij����Һ����KMnO4����Һ�ζ�ʱӦѡ���� �� �����ζ��ܣ����ʽ����ʽ������
��4�����ⶨ��ҺA��Fe2+��Ũ�ȣ���Ҫ������ƿ����ij����Һ����KMnO4����Һ�ζ�ʱӦѡ���� �� �����ζ��ܣ����ʽ����ʽ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 �������ϣ���Cu2O���ڼ�������� ���ڿ���������Cu2O����CuO����Cu2O�������������ܷ������з�Ӧ��Cu2O��2H+��Cu��Cu2+��H2O��
�������ϣ���Cu2O���ڼ�������� ���ڿ���������Cu2O����CuO����Cu2O�������������ܷ������з�Ӧ��Cu2O��2H+��Cu��Cu2+��H2O�� �鷽����
�鷽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ����� | ʵ������ | ʵ����� |
| �ò���Ƭ��ˮ���е��Թܿڸ�ס�����Ὣ�Թ�ȡ�� | �Թ���Լ��2/3�����ˮ��Һ������ɫ | NO2��ˮ�����˻�ѧ��Ӧ������������ˮ |
| ���Թ�������ȡ�²���Ƭ | �� | NO2��ˮ��Ӧ������NO���� |
| �� | �� | NO2��ˮ��Ӧ���������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com