���𰸡�
���������ȷֶη���ͼ����ÿ��ͼ������ʾ����Һ�����ʳɷ���ʲô��Ȼ���Ϸ���ʽ��ԭ���غ����NaOH������������P����Һ�е����ʳɷ֣��ٴν�Ϸ���ʽ���������������������ʵ���������������������
�����ÿһ��ʵ����������ӹ����ж������Ƿ���ڣ�
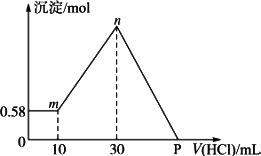
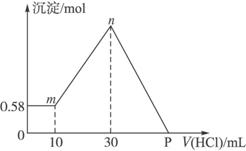
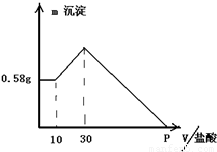
����⣺����������Һ�У���μ���1mol/L���ᣬ�ɼ����������������ɳ�����������ϵͼ������
��0-10ml���������������ӣ����ɳ������������䣬˵��������Mg��OH��
2��m[Mg��OH��
2]=0.58g��NaOH��AlCl
3��MgCl
2��ɵĻ�����������ˮ������Ӧ�ǣ�MgCl
2+2NaOH=Mg��OH��
2��+2NaCl��AlCl
3+4NaOH=NaAlO
2+3NaCl+2H
2O��NaOH��ʣ�࣬��Һ��NaCl��NaAlO
2��NaOH�Ļ��Һ���ýη�����Ӧ�ǣ�NaOH+HCl=NaCl+H
2O��
��10ml��������10ml����պ��к�δ��Ӧ��NaOH����ҺΪNaCl��NaAlO
2��
��10ml-30ml���������������ӣ����ɳ������������ӣ��ýη�����Ӧ�ǣ�NaAlO
2+HCl+H
2O=Al��OH��
3��+NaCl��
��30ml����NaAlO
2������ǡ�÷�Ӧ�����������Ϊ��30ml-10ml=20ml�����������ﵽ�����ҺΪNaCl��Һ��
��30ml-p�㣬�������������ӣ��������������٣�������Ӧ�ǣ�Mg��OH��
2+2HCl=MgCl
2+2H
2O��
Al��OH��
3+3 HCl=AlCl
3+3H
2O��
��p�㣬Mg��OH��
2��Al��OH��
3��ȫ��Ӧ����ҺΪMgCl
2��AlCl
3��NaCl���Һ��
ѡ30ml��������NaOH����������ʱ����ҺΪNaCl��Һ����Һ��Cl
-��Դ��ԭ������е�AlCl
3��MgCl
2�ͼ����30mlHCl����Һ��Na
+��Դ��ԭ������е�NaOH��
NaAlO
2 +HCl+H
2O=Al��OH��
3��+NaCl
0.02mol 0.02L×1mol/L=0.02mol
��Alԭ���غ��ԭ�������n��AlCl
3��=n��NaAlO
2��=0.02mol
��Mgԭ���غ��ԭ�������n��MgCl
2��=n[Mg��OH��
2]=
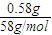
=0.01mol��
��Na
+���Ӻ�Cl
-�����غ�ã�
n��NaOH��=n��NaCl��=n��Cl
-��=2n��MgCl
2��+3n��AlCl
3��+n��HCl��=0.01mol×2+0.02mol×3+0.03L×1mol/L=0.11mol
���ԣ�ԭ�������NaOH��������m��NaOH��=0.11mol×40g/mol=4.4g
P����ҺΪMgCl
2��AlCl
3��NaCl���Һ��P��������������������к�ԭ������е�NaOH����ʱ�����������ʵ�����
n��HCl��=n��NaOH��=0.11mol��P������ʾ��������Ϊ��V=
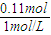
=0.11L=110ml
�ʴ�Ϊ����1��4.4g��2��110ml
��ij����Һ���ܺ���Ag
+��Fe
3+��K
+��Ba
2+��NH
4+��SO
42-��NO
3-�����ӣ���������ʵ�飺
�ټ��������ϡ���ᣬ�а�ɫ�������ɣ�˵��ԭ��Һ��һ������Ag
+��һ������SO
42-����ΪAg
+��SO
42-��ϳ�����
Ag
2SO
4����Һ�ʵ����ԣ�һ��������NO
3-��
�ڹ��ˣ�����Һ�м��������ϡ���ᣬ���а�ɫ�������ɣ�˵��ԭ��Һ��һ������Ba
2+��
�۹��ˣ�ȡ������Һ������2��KSCN��Һ��û�����Ե�������֣�˵��ԭ��Һ��һ��û��Fe
3+��
����ȡ����������е���Һ������NaOH��Һ��ʹ��Һ�ʼ��ԣ����ȣ��ɲ���ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壮˵��ԭ��Һ��һ������NH
4+��
K
+��ȷ������ͨ����ɫ��Ӧ������ɫ�ܲ�������һ��ȷ����
�ʴ�Ϊ��Ag
+��Ba
2+��NH
4+��NO
3-�� SO
42-��Fe
3+���������ڱ����ͼ��Ҫ��ȷÿ��ͼ�����ķ���ʽ��֪���յ��ʾ�ĺ��壬֪��ÿ���յ��ʾ����Һ�����ʵijɷ֣�ֻ��Ū�����Щ��������ȷ�Ľ�����⣮

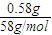 =0.01mol��
=0.01mol��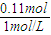 =0.11L=110ml
=0.11L=110ml

 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�


![]() Al(OH)3����NaCl��H2O)
Al(OH)3����NaCl��H2O)