
 E�����ڱ��е�λ�õ������ڵ�VIIA��
E�����ڱ��е�λ�õ������ڵ�VIIA��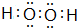
| ѡ�� | a | b | c | d |
| X | �¶� | �������� | ����� | �¶� |
| Y | n������ | ����ת���� | n������� | n��H2�� |
���� A��B��C��D��E��ԭ��������������Ķ�����Ԫ�أ���3����B�������̬�⻯�����ˮ��Һ�Լ��ԣ���BΪNԪ�أ���ΪNH3��C�ǵؿ��к�����ߵĽ�������CΪAl��D��C�����γ�C2D3�͵Ļ������������D����-2�ۣ���DΪS��E��ԭ���������EΪCl��A�������������̬�⻯���������Ԫ�ص������ٷֺ���Ϊ��ߵ����ʣ�ԭ������С�ڵ�Ԫ�أ���AΪ̼Ԫ�أ�
��1��C������ΪAl3+���ӣ�������Ϊ13�����������Ϊ10����2�����Ӳ㣬����������Ϊ8������Ԫ��������=���Ӳ���������������=������������
��2��A����Ԫ����ɵĻ�������Ӽ��к���8��ԭ�ӣ���ΪC2H6����Dͬ�����ijԪ��������ɵĻ���������ĵ�������ȣ�����ΪH2O2��
��3���ٱ���ˮ��Һ���ҷ�Ӧ�IJ��ﲻ��Ⱦ������Ӧ���ɵ�����ˮ��
�ڷ����ֽⷴӦ��2NH3?N2+3H2 ��H��0������ƽ���ƶ�ԭ���������
��4��Al3+��S2-z��ˮ��Һ�з���ǿ��˫ˮ������Al��OH��3��H2S��
��5��������Ӧ��CS2+3O2=CO2+2SO2��ע�����ʾۼ�״̬�뷴Ӧ����д�Ȼ�ѧ����ʽ��
��� �⣺A��B��C��D��E��ԭ��������������Ķ�����Ԫ�أ���3����B�������̬�⻯�����ˮ��Һ�Լ��ԣ���BΪNԪ�أ���ΪNH3��C�ǵؿ��к�����ߵĽ�������CΪAl��D��C�����γ�C2D3�͵Ļ������������D����-2�ۣ���DΪS��E��ԭ���������EΪCl��A�������������̬�⻯���������Ԫ�ص������ٷֺ���Ϊ��ߵ����ʣ�ԭ������С�ڵ�Ԫ�أ���AΪ̼Ԫ�أ�
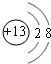
��1��C������ΪAl3+���ӣ�������Ϊ13�����������Ϊ10����2�����Ӳ㣬����������Ϊ8�����ӽṹʾ��ͼΪ ��EΪClԪ�أ������ڱ���λ�ڵ������ڵ�VIIA�壬�ʴ�Ϊ��
��EΪClԪ�أ������ڱ���λ�ڵ������ڵ�VIIA�壬�ʴ�Ϊ�� ���������ڵ�VIIA�壻
���������ڵ�VIIA�壻
��2��A����Ԫ����ɵĻ�������Ӽ��к���8��ԭ�ӣ���ΪC2H6����Dͬ�����ijԪ��������ɵĻ���������ĵ�������ȣ�����ΪH2O2������ʽΪ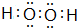 ���ʴ�Ϊ��
���ʴ�Ϊ��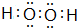 ��
��
��3���ٱ���ˮ��Һ���ҷ�Ӧ�IJ��ﲻ��Ⱦ������Ӧ���ɵ�����ˮ����Ӧ����ʽΪ2NH3•H2O+3H2O2=N2��+8H2O���ʴ�Ϊ��2NH3•H2O+3H2O2=N2��+8H2O��
�ڷ����ֽⷴӦ��2NH3?N2+3H2 ��H��0��
a���÷�Ӧ�����ȷ�Ӧ���¶����ߣ�ƽ��������Ӧ�ƶ���NH3�����ʵ�����С����a���ϣ�
b������H2��������Ũ������ƽ�����淴Ӧ���У�NH3��ת���ʽ��ͣ���b���ϣ�
c������NH3��ƽ��������Ӧ�ƶ����ܵ����ʵ�������c�����ϣ�
d���÷�Ӧ�����ȷ�Ӧ���¶����ߣ�ƽ��������Ӧ�ƶ���H2�����ʵ�������d�����ϣ�
�ʴ�Ϊ��ab��
��4��Al3+��S2-��ˮ��Һ�з���ǿ��˫ˮ������Al��OH��3��H2S������ͨ�����ֽⷴӦ�ϳ�Al2S3��
�ʴ�Ϊ�����ܣ�Al3+��S2-��ˮ��Һ�з���ǿ��˫ˮ������Al��OH��3��H2S��
��5��Һ̬������CS20.2mol��O2����ȫȼ�գ�����������̬������ΪCO2��SO2��298Kʱ�ų�����215kJ����1molCS2��ȫȼ�շų�������Ϊ215kJ��$\frac{1mol}{0.2mol}$=1075kJ���÷�Ӧ���Ȼ�ѧ����ʽΪCS2��l��+3O2��g��=CO2��g��+2SO2��g����H=-1075kJ/mol��
�ʴ�Ϊ��CS2��l��+3O2��g��=CO2��g��+2SO2��g����H=-1075kJ/mol��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��漰���û�ѧ�������ˮ�⡢Ӱ�컯ѧƽ������ء��Ȼ�ѧ����ʽ��д�ȣ���Ŀ��Ϊ�ۺϣ��Ѷ��еȣ�ע��Ի���֪ʶ��ȫ���������գ��ƶ�Ԫ���ǹؼ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��1����ѧ�Ƶ�ԭ�������û�ѧ��Ӧ���ɽ������ʳ����ڶƼ������γɶƲ㣮
��1����ѧ�Ƶ�ԭ�������û�ѧ��Ӧ���ɽ������ʳ����ڶƼ������γɶƲ㣮
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ������Ԫ��A��B��C��D��Ԫ�����ڱ��е����λ����ͼ��ʾ������B������������������������ȣ������жϴ�����ǣ�������
������Ԫ��A��B��C��D��Ԫ�����ڱ��е����λ����ͼ��ʾ������B������������������������ȣ������жϴ�����ǣ�������| A�� | ԭ�Ӱ뾶��B��C��A | |
| B�� | ����������Ӧˮ��������ԣ�C��D | |
| C�� | ��BԪ�ص�����Һһ�������� | |
| D�� | �����̬�⻯������ȶ��ԣ�A��C |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ۺ���ά�ص�ͨʽ���ǣ�C6H10O5��n����ͬ���칹�� | |
| B�� | ������Һ�м��루NH4��2SO4������Һ���г����������ټ�ˮ�������ܽ� | |
| C�� | ��֬������͵�������ʳ�ﺬ�е���ҪӪ�����ʣ����Ƕ��Ǹ߷��ӻ����� | |
| D�� | ���������Ƶ�Cu��OH��2����Һ���黼�ߵ���Һ���Ƿ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ʵ�����е�CO2�����ô���ʯ��ϡ���ᷴӦ��ȡ��������ͼװ�ý����ᴿ���
ʵ�����е�CO2�����ô���ʯ��ϡ���ᷴӦ��ȡ��������ͼװ�ý����ᴿ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
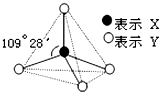 �ס��ҡ����������������ͼ��ʾ�Ľṹ��ṹ��Ԫ��ͼ��������������еIJ���δ������ֻ��ʵ�߱�ʾ���ۼ���X��Y��ͬ�ɲ�ͬ��
�ס��ҡ����������������ͼ��ʾ�Ľṹ��ṹ��Ԫ��ͼ��������������еIJ���δ������ֻ��ʵ�߱�ʾ���ۼ���X��Y��ͬ�ɲ�ͬ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ������ | K+ Na+ Cu2+ Al3+ |
| ������ | SO42- HCO3-NO3- OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ�ʵ����ԣ�������е�� | |
| B�� | ��Һ��������һ�����磬�����з�ɢ�����Ӵ��磬��ͨ������������������ƶ�������������һ���ƶ� | |
| C�� | ��Һ�����������й����˶������������������˶� | |
| D�� | ��Һ��ͨ��һ������ʱ��������������ͨ��һ����ʱ�����ԵĹ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ba��OH��2��Һ�еμ�ϡ���Ba2++2OH-+2H++SO42-�TBaS04��+2H2O | |
| B�� | ���Խ�����KMnO4���� H2O2��2MnO4-+5H2O2+6H+�T2Mn2++5O2��+8H2O | |
| C�� | �����ʵ�����MgCl2��Ba��OH��2 �� HCl ��Һ��ϣ�Mg2++2OH-�TMg��OH��2�� | |
| D�� | Ǧ�����س��ʱ��������Ӧ��PbSO4+2H2O-2e-�TPbO2+4H++SO42- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com