
����Ŀ����14�֣���ѧʵ�鳣����ͼ��ʾװ����ȡ�����屽������ƿa��װ���Լ��DZ���������ۡ�����д���пհס�
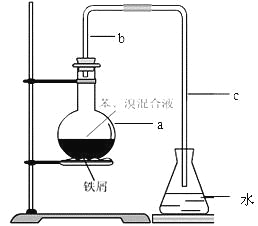
������b��������������һ�ǵ��������Ǽ��� �����ã�
��a�з�Ӧ�Ļ�ѧ����ʽΪ ��
����ʵ��õ����Ǵֱ���Ϊ�˳�ȥ���е��壬Ҫ�õ���һ�ֳ��ó��Ӳ��������� ���ᴿ�屽�ľ��岽���У�
��������ˮϴ�����ø���������10%NaOH��Һϴ����ˮϴ ��
��ȷ�IJ���˳��Ϊ�� ��
A���٢ڢۢܢ� | B���ڢܢݢۢ� | C���ܢڢۢ٢� | D���ڢܢ٢ݢ� |
��Ϊ֤�������巢������ȡ����Ӧ�����Ǽӳɷ�Ӧ������ˮ�м��� ������ʵ�鲻�����ܣ�Ϊ�˱����ڵ���a��b֮�����һʢ�� ��ϴ��ƿ��Ŀ���� ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ס���Ϊͬ����Ԫ�أ��ش��������⣺
��1����̬Asԭ�ӵĺ�������Ų�ʽΪ_______��
��2���ص���Ͻ���ϵ�̫���ܵ��Ч�ʴ�40%����3��Ԫ�صĵ縺���ɴ���С��˳����______ ����Ԫ�ط��ű�ʾ��
��3��As4O6�ķ��ӽṹ������ͼ��ʾ������Asԭ�ӵ��ӻ���ʽΪ______________��1 mol As406�������������ʵ���Ϊ_______��


��4�������⻯��RH3(NH3��PH3��AsH3)��ij��������R�ĺ˵�����ı仯����������ͼ��ʾ����Y��ɱ�ʾ���⻯�RH3�����ʿ�����__________��
A.�ȶ��� B.�е� C.R-H���� D.���Ӽ�������
��5��AsH3�ķе㣨-62.5�棩��NH3�ķе㣨-33.5�棩�ͣ�ԭ����_____________________
��6��NH4+�е�H��N��H�ļ��DZ� NH3�е�H��N��H�ļ���_______�����С������ԭ����_______________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о�С����̽��SO2�Ļ�ѧ���ʣ����������ʵ�鷽����
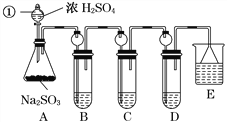
(1)ָ�������������ƣ�______________��
(2)���Aװ�õ������Եķ�����______________________________________________��
(3)װ��B����SO2�������ԣ���B����ʢ�Լ�����Ϊ________��
(4)װ��C��ʢװ��ˮ���Լ���SO2��________�ԣ���C�з�Ӧ�����ӷ���ʽΪ
________________________________________________________________________��
(5)װ��D��ʢװ����Ư��Ũ��Һ��ͨ��SO2һ��ʱ���D�г����˴�����ɫ������ͬѧ�Ƕ�ɫ�����ɷ�������ּ��裺
������һ���ð�ɫ����ΪCaSO3��
��������ð�ɫ����Ϊ__________________________________________________��
���������ð�ɫ����Ϊ�����������ʵĻ���
�����ڼ���һ��ͬѧ�Ƕ�ɫ�����ɷֽ�����̽����������·�����
��ѡ���������Լ�������װ�á��Թܡ��ιܡ������ܵĵ�����������ˮ��0.5 mol��L��1HCl��0.5 mol��L��1H2SO4��0.5 mol��L��1BaCl2��1 mol��L��1NaOH��Ʒ����Һ��
��1������D�г������ˡ�ϴ�Ӹɾ������á�
��ش�ϴ�ӳ����ķ�����____________________________________________________��
��2��������һֻ�ɾ��Թ�ȡ����������Ʒ������________(�Լ�)�����ϴ����ܵĵ������������ܵ���һ�˲���ʢ��________(�Լ�)���Թ��С�������__________________���������һ������
�����������������д�����ɸð�ɫ�����Ļ�ѧ����ʽ��_________________________________��
(6)װ��E��ʢ�ŵ��Լ���________��������__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������¶���ڿ������ױ��ʵ���(����)
����ˮ ��ˮ���� ���ռ� ��Ư�� ���̷� ���������� ��Ũ���� ��Ũ����
A. �٢ڢۢܢޢ� B. �٢ݢޢ� C. �ܢݢߢ� D. �٢ڢۢܢݢޢߢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Կ��淴Ӧ:A(g)+B(s)![]() C(s)+D(g)����H>0����ͼ��ʾΪ�����淴Ӧ����(v)��ʱ��(t)��ϵ��ʾ��ͼ,�����t1ʱ�̸ı���������:������A;���������;����ѹ;������;������C,����ͼʾ��������(����)
C(s)+D(g)����H>0����ͼ��ʾΪ�����淴Ӧ����(v)��ʱ��(t)��ϵ��ʾ��ͼ,�����t1ʱ�̸ı���������:������A;���������;����ѹ;������;������C,����ͼʾ��������(����)
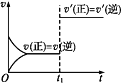
A. �ڢ� B. �٢� C. �ۢ� D. �ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.һ�������������Ժ�CO2������Ӧ:Fe(s)+CO2(g)![]() FeO(s)+CO(g)����H>0��
FeO(s)+CO(g)����H>0��
(1)���д�ʩ����ʹƽ��ʱ![]() �������____(�����)��
�������____(�����)��
A.�����¶� B.����ѹǿ C.����һ�������� D.�ټ���һЩ����
(2)��Ӧ�ﵽƽ���,�����������������ʱ,��ͨ��һ������CO2,ʹCO2��Ũ�ȳ�Ϊԭ����2��,��CO2��ת���ʽ�____(��������������С������������)��
��.��һ���¶��µ�ij�ݻ�������ܱ�������,�������л�ѧƽ��:C(s)+H2O(g)![]() CO(g)+H2(g),�Է����ͻش���������:
CO(g)+H2(g),�Է����ͻش���������:
(1)���϶��������淴Ӧ��һ���������Ѵﵽ��ѧƽ��״̬����____(ѡ�����)��
A.��ϵ��ѹǿ���ٷ����仯
B.v��(CO)=v��(H2O)
C.����n mol CO��ͬʱ����n mol H2
D.1 mol H��H�����ѵ�ͬʱ����2 mol H��O��
E.���������ܶ�
(2)���������ݻ��ɱ䣬������ѧƽ��״̬������Ӧ��ʼ����,�ﵽƽ���,��ƽ����ϵ��ѹ(��С�ݻ���������������),�������������ƽ����Է���������____(��������������С�����������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����Ϊ��Ԫ���
H2C2O4![]() HC2O4��+H+ Ka1
HC2O4��+H+ Ka1
HC2O4��![]() C2O42��+H+ Ka2
C2O42��+H+ Ka2
����������ijŨ�ȵIJ�����Һ����μ���һ����Ũ�ȵ�KOH��Һ��������Һ��H2C2O4��HC2O4����C2O42�������������ʵ�����������������ҺpH�Ĺ�ϵ��ͼ��ʾ��������˵��������ȷ����

A��pH=1.2��Һ����c(K+) + c(H+) = c(OH��) + c(H2C2O4)
B��pH=2.7��Һ����c2(HC2O4��) / [c(H2C2O4) �� c(C2O42��)]=1000
C������ͬ���ʵ���KHC2O4��K2C2O4������ȫ����ˮ�����pHΪ4.2�Ļ��Һ
D����pH=1.2����Һ�м�KOH��Һ��pH������4.2�Ĺ�����ˮ�ĵ����һֱ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�������������������˵���������
A. �����е��ޡ��顢��˿���պ�IJ��ﲻͬ
B. ��������ϩ���Ͽ����¼ӹ��ɷ�ˮ���ϻ����͵�ȼ�ϣ����Ϊ��
C. �������õ���̼��ά���ά��Ϊ����
D. ֬�����л�����֯��洢��������Ҫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���������5000����õ����������й���ѧ��ԭʼ��ʽ��
A. ������ B. ��ֽ�� C. �ƻ�ҩ D. �˿��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com