
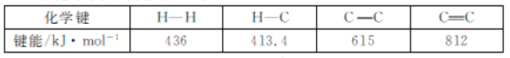
ΓΨΧβΡΩΓΩ““»≤Φ”«β «““œ©ΙΛ“Β÷–ΒΡ÷Ί“ΣΨΪ÷ΤΖ¥”ΠΘ§άϊ”Ο’β“ΜΖ¥”ΠΩ…“‘ΫΪ““œ©≤ζΤΖ÷–ΒΡ““»≤Κ§ΝΩΫΒΒΆΘ§“‘±ήΟβΚσ–χ““œ©ΨέΚœ¥ΏΜ·ΦΝΒΡ÷–ΕΨΘ§ΙΛ“Β…œ≥ΤΈΣΧΦΕΰΦ”«βΙΐ≥ΧΓΘ
“―÷ΣΘΚΔώ.CH![]() CH(g)+H2(g)ΓζCH2=CH2(g) ΠΛH1 K1(400K)=4.2ΓΝ1022
CH(g)+H2(g)ΓζCH2=CH2(g) ΠΛH1 K1(400K)=4.2ΓΝ1022
Δρ.CH![]() CH(g)+2H2(g)ΓζCH3CH3(g) ΠΛH2=-311.4kJΓΛmol-1 K2(400K)=1.4ΓΝ1038
CH(g)+2H2(g)ΓζCH3CH3(g) ΠΛH2=-311.4kJΓΛmol-1 K2(400K)=1.4ΓΝ1038
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©“―÷ΣΦΗ÷÷Μ·―ßΦϋΒΡΦϋΡή»γœ¬±μΥυ ΨΘΚ
ΓςH1=___kJmol-1ΓΘ
Θ®2Θ©400K ±Θ§‘ΎΟή±’»ίΤς÷–ΫΪΒ»Έο÷ ΒΡΝΩΒΡCH2=CH2(g)ΚΆH2(g)ΜλΚœΘ§≤…”Ο Β±ΒΡ¥ΏΜ·ΦΝΫχ––Ζ¥”ΠΘ§…ζ≥…CH3CH3(g)Θ§¥οΒΫΤΫΚβ ±≤βΒΟ![]() =1016Θ§‘ρΤΫΚβ ±c(H2)=___molL-1ΓΘ
=1016Θ§‘ρΤΫΚβ ±c(H2)=___molL-1ΓΘ
Θ®3Θ©Ψί«Α»Υ―–ΨΩΖΔœ÷““»≤‘ΎPVΆ≈¥Ί±μΟφ¥ΏΜ·Φ”«βΖ¥”ΠΒΡ≤ΩΖ÷άζ≥Χ»γΆΦ1Υυ ΨΘ§Τδ÷–ΈϋΗΫ‘ΎPV±μΟφ…œΒΡΈο÷÷”Ο*±ξΉΔΓΘ

ΆΤ≤β““œ©‘ΎPV±μΟφ…œΒΡΈϋΗΫΈΣ___(ΧνΓΑΖ≈»»Γ±ΜρΓΑΈϋ»»Γ±Θ©Ιΐ≥ΧΓΘΆΦ1άζ≥Χ÷–Ήν¥σΡήΫπΘ®ΜνΜ·ΡήΘ©E’ΐ=___kJΓΛmol-1Θ§ΗΟ≤Ϋ÷ηΒΡΜ·―ßΖΫ≥Χ ΫΈΣ___ΓΘ
Θ®4Θ©T1Γφ ±Θ§ΫΪΧεΜΐ±»ΈΣ1ΘΚ2ΒΡCHΓ‘CH(g)ΚΆH2(g)≥δ»κΗ’–‘Οή±’»ίΤς÷–Θ§Φ”»κ¥ΏΜ·ΦΝΖΔ…ζΖ¥”ΠΔρΘ§Τπ ΦΧεœΒΉή―Ι«ΩΈΣP0 kPaΘ§ Β―ι≤βΒΟH2ΒΡΖ÷―ΙΘ®p)”κΖ¥”Π ±ΦδΘ®tΘ©ΒΡΙΊœΒ»γΆΦ2Υυ ΨΓΘ
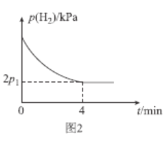
ΔΌT1Γφ ±Θ§0ΓΪ4minΡΎΘ§ΤΫΨυΖ¥”ΠΥΌ¬ v(HCΓ‘CH)=___kPamin-1(”ΟΚ§p0ΓΔp1ΒΡ¥ζ ΐ Ϋ±μ ΨΘ§œ¬Ά§Θ©ΓΘ
ΔΎT1Γφ ±Θ§ΗΟΖ¥”ΠΒΡΜ·―ßΤΫΚβ≥Θ ΐKp=___kPa-2(KpΈΣ“‘Ζ÷―Ι±μ ΨΒΡΤΫΚβ≥Θ ΐΘ§Ζ÷―Ι=Ήή―ΙΓΝΈο÷ ΒΡΝΩΖ÷ ΐΘ©ΓΘ
ΔέT1Γφ ±Θ§0ΓΪ2minΡΎp(H2)ΒΡΦθ–ΓΝΩ___(ΧνΓΑΘΨΓ±ΓΑΘΦΓ±ΜρΓΑ=Γ±Θ©2ΓΪ4minΡΎp(H2)ΒΡΦθ–ΓΝΩΘ§άμ”…ΈΣ___ΓΘ
ΓΨ¥πΑΗΓΩ-193.8 3 Ζ≈»» 22.59 C2H3+ H= C2H4+ ![]()
![]() ΘΨ Ζ¥”ΠΈοΒΡ≈®Ε»Φθ–ΓΘ§Ζ¥”ΠΥΌ¬ Φθ¬ΐ
ΘΨ Ζ¥”ΠΈοΒΡ≈®Ε»Φθ–ΓΘ§Ζ¥”ΠΥΌ¬ Φθ¬ΐ
ΓΨΫβΈωΓΩ
(1)ΫαΚœ±μΗώ÷–ΦϋΡή ΐΨίΘ§ΗυΨίΓςH=Ζ¥”ΠΈοΒΡΦϋΡή -…ζ≥…ΈοΒΡΦϋΡήΫχ––Ϋβ¥πΘΜ
(2)‘Υ”ΟΗ«ΥΙΕ®¬…Ϋβ≥ωΡΩ±ξΖ¥”ΠΘ§ΗυΨί“―÷ΣΖ¥”Π”κΡΩ±ξΖ¥”ΠΒΡΤΫΚβ≥Θ ΐΙΊœΒΦΤΥψΘΜ
(3)ΫαΚœΆΦ÷–ΗςΈο÷ Ζ¥”ΠΙΊœΒ÷–Ζ¥”ΠΈο…ζ≥…ΈοΡήΝΩ±δΜ·Ζ÷Έω≈–ΕœΘΜ
(4)άϊ”ΟΓΑ»ΐΕΈ ΫΦΤΥψΓ±Ζ¥”ΠΥΌ¬ ΓΔΤΫΚβ≥Θ ΐΘΜΖ¥”ΠΙΐ≥Χ÷–Ζ¥”ΠΈοΒΡ≈®Ε»±δΜ·Ζ÷ΈωΘΜ
(1)ΗυΨίΓςH=Ζ¥”ΠΈοΒΡΦϋΡή -…ζ≥…ΈοΒΡΦϋΡήΘ§‘ρΠΛH1=413.4 kJmol-1ΓΝ2+812 kJmol-1+436 kJmol-1-(413.4 kJmol-1ΓΝ4+615 kJmol-1)=-193.8 kJmol-1ΘΜ
(2)“―÷ΣΘΚΔώ.CH![]() CH(g)+H2(g)ΓζCH2=CH2(g) ΠΛH1 K1(400K)=4.2ΓΝ1022
CH(g)+H2(g)ΓζCH2=CH2(g) ΠΛH1 K1(400K)=4.2ΓΝ1022
Δρ.CH![]() CH(g)+2H2(g)ΓζCH3CH3(g) ΠΛH2=-311.4kJΓΛmol-1 K2(400K)=1.4ΓΝ1038
CH(g)+2H2(g)ΓζCH3CH3(g) ΠΛH2=-311.4kJΓΛmol-1 K2(400K)=1.4ΓΝ1038
ΗυΨίΗ«ΥΙΕ®¬…Θ§Δρ-ΔώΒΟΘΚCH2=CH2(g)+H2(g)ΓζCH3CH3(g)Θ§ΤΫΚβ ±≤βΒΟ![]() =1016Θ§ΤΫΚβ≥Θ ΐK=
=1016Θ§ΤΫΚβ≥Θ ΐK=![]() =
=![]() =
=![]() Θ§‘ρc(H2)=3mol/LΘΜ
Θ§‘ρc(H2)=3mol/LΘΜ
(3)»γΆΦ1Υυ ΨΘ§IM6ΈΣC2H4Θ§C2H4ΓζC2H4ΒΡΙΐ≥ΧΈΣ““œ©ΒΡΆ―ΗΫΙΐ≥ΧΘ§–η“ΣΈϋ ’14.58 kJmol-1ΒΡΡήΝΩΘ§‘ρΖ¥÷°C2H4ΓζC2H4ΒΡΙΐ≥ΧΈΣ““œ©ΒΡΈϋΗΫΙΐ≥ΧΘ§Ζ≈≥ω14.58 kJmol-1ΒΡΡήΝΩΘΜ»γΆΦΥυ ΨΘ§IM4ΓζIM5Ιΐ≥Χ÷–ΜνΜ·ΡήΉν¥σΘ§ΈΣ-32.33 kJmol-1-(-54.92 kJmol-1)=22.59 kJmol-1Θ§ΗΟ≤Ϋ÷ηΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ ΫΈΣΘΚC2H3+ H= C2H4+ ΘΜ
(4)ΔΌΗυΨίΖ¥”ΠΘΚCH![]() CH(g)+2H2(g)ΓζCH3CH3(g)Θ§»γΆΦΥυ ΨΘ§ΩΣ Φ ±p(CH
CH(g)+2H2(g)ΓζCH3CH3(g)Θ§»γΆΦΥυ ΨΘ§ΩΣ Φ ±p(CH![]() CH)=
CH)=![]() kPaȧp(H2)=
kPaΘ§p(H2)=![]() kPaΘ§ΗυΨίΓΑ»ΐΕΈ ΫΓ±ΘΚ
kPaΘ§ΗυΨίΓΑ»ΐΕΈ ΫΓ±ΘΚ

‘ρ2x=![]() -
-![]() Θ§ΫβΒΟx=
Θ§ΫβΒΟx=![]() - p1Θ§Ι 0-4minΡΎΘ§ΤΫΨυΖ¥”ΠΥΌ¬ v(HCΓ‘CH)=
- p1Θ§Ι 0-4minΡΎΘ§ΤΫΨυΖ¥”ΠΥΌ¬ v(HCΓ‘CH)=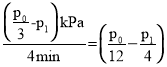 kPamin-1ΘΜ
kPamin-1ΘΜ
ΔΎ”…ΔΌΒΡΓΑ»ΐΕΈ ΫΓ±Ω…ΒΟKp=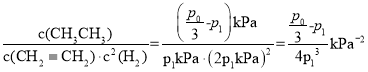 ΘΜ
ΘΜ
ΔέΥφΉ≈Ζ¥”ΠΒΡΫχ––Θ§Ζ¥”ΠΈοΒΡ≈®Ε»Φθ–ΓΘ§Ζ¥”ΠΥΌ¬ Φθ¬ΐΘ§‘ρ0ΓΪ2minΡΎ«βΤχΒΡ±δΜ·ΝΩ¥σ”Ύ2ΓΪ4minΡΎΘ§‘ρ0ΓΪ2minΡΎp(H2)ΒΡΦθ–ΓΝΩΘΨ2ΓΪ4minΡΎp(H2)ΒΡΦθ–ΓΝΩΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–»ή“Κ÷–ΗςΈΔΝΘΒΡ≈®Ε»ΙΊœΒ“ΜΕ®’ΐ»ΖΒΡ «Θ® Θ©
A.25Γφ ±Θ§pHΨυΈΣ10ΒΡΑ±Υ°”κ BaΘ®OHΘ©2 »ή“Κ÷–Θ§»ή÷ ΒΡΈο÷ ΒΡΝΩ≈®Ε»÷°±»ΈΣ1:2
B.Ρ≥Έ¬Ε»œ¬Θ§0.1molΓΛL1 NaHCO3»ή“ΚΒΡpH=8Θ§‘ρ cΘ®OHΘ©=106molΓΛL1
C.0.1molΓΛL1ΒΡHA»ή“ΚΒΡpH=2Θ§»ή“Κ÷–cΘ®H+Θ©=cΘ®OHΘ©ΘΪcΘ®AΘ©
D.pH=9ΒΡCH3COONa»ή“Κ”κpH=9ΒΡΑ±Υ°÷–Θ§Υ°Βγάκ≥ωΒΡcΘ®OHΘ©÷°±»ΈΣ1:1
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ≥ΘΈ¬œ¬Θ§œρ20 mL 0.1 molLΘ≠1ΒΡMOH»ή“Κ÷–÷πΒΈΦ”»κ0.1 molLΘ≠1 HCl»ή“ΚΘ§ΒΈΕ®«ζœΏ»γΆΦΥυ ΨΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
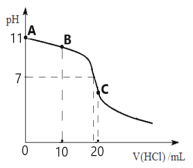
Θ®1Θ©«κ–¥≥ωMOHΒΡΒγάκΖΫ≥Χ Ϋ__________________________Θ§≥ΘΈ¬œ¬Θ§ΗΟΦνΒΡΒγάκΤΫΚβ≥Θ ΐKb=_________ΓΘ
Θ®2Θ©ΒΈΕ®ΒΫBΒψ ±Θ§»ή“Κ÷–ΗςάκΉ”≈®Ε»”…¥σΒΫ–ΓΒΡΥ≥–ρ «_________________________ΓΘ
Θ®3Θ©CΒψΥυΕ‘”ΠΒΡ»ή“Κ≥ _____–‘Θ§”ΟάκΉ”ΖΫ≥Χ Ϋ±μ ΨΤδ‘≠“ρ______________________ΓΘ
Θ®4Θ©ΆΦ÷–AΓΔBΓΔC»ΐΒψ»ή“Κ÷–Υ°ΒΡΒγάκ≥ΧΕ»”…¥σΒΫ–ΓΒΡΥ≥–ρ «_______________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩCOΓΔH2 «ΟΚΒΡΤχΜ·≤ζΈοΘ§‘Ύ…ζ≤ζ…ζΜν÷–”ΟΆΨΙψΖΚΓΘ
Θ®1Θ©COΜΙ‘≠Ζ®¥Πάμ¥σΤχΈέ»ΨΈοSO2
ΔΌ2COΘ®gΘ© + SO2Θ®gΘ© ![]() SΘ®sΘ©+2CO2Θ®gΘ© H = -270 kJΓΛmolΘ≠1Θ§ΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐ±μ¥ο ΫΈΣ__ΓΘ
SΘ®sΘ©+2CO2Θ®gΘ© H = -270 kJΓΛmolΘ≠1Θ§ΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐ±μ¥ο ΫΈΣ__ΓΘ
ΔΎ‘ΎΨχ»»Κψ»ίΒΡΟή±’»ίΤς÷–Ϋχ––…œ ωΖ¥”ΠΘ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «_____ΓΘ
a »τΜλΚœΤχΧεΟήΕ»±Θ≥÷≤Μ±δΘ§‘ρ“―¥οΤΫΚβΉ¥Χ§
b ¥οΤΫΚβΚσ»τ‘Ό≥δ»Υ“ΜΕ®ΝΩCO2Θ§ΤΫΚβ≥Θ ΐ±Θ≥÷≤Μ±δ
c Ζ÷άκ≥ω≤ΩΖ÷SΘ§’ΐΓΔΡφΖ¥”ΠΥΌ¬ Ψυ±Θ≥÷≤Μ±δΘ§ΤΫΚβ≤Μ“ΤΕ·
d ¥”Ζ¥”ΠΩΣ ΦΒΫΤΫΚβΘ§»ίΤςΡΎΤχΧεΒΡ―Ι«Ω±Θ≥÷≤Μ±δ
Δέœρ2 LΚψΈ¬Κψ»ίΟή±’»ίΤς÷–Ά®»Υ2 mol COΓΔ1 mol SO2Θ§Ζ÷±πΫχ––aΓΔbΓΔc»ΐΉι Β―ιΓΘ‘Ύ≤ΜΆ§¥ΏΜ·ΦΝΦΰœ¬ΖΔ…ζΖ¥”ΠΘΚ2COΘ®gΘ© + SO2Θ®gΘ© ![]() SΘ®sΘ©+2CO2Θ®gΘ© H = -270 kJΓΛmolΘ≠1Θ§Ζ¥”ΠΧεœΒΉή―ΙΥφ ±ΦδΒΡ±δΜ·»γœ¬±μΥυ ΨΘ§‘ρ»ΐΉι Β―ιΈ¬Ε»ΒΡ¥σ–ΓΙΊœΒ «_____Θ®”ΟaΓΔbΓΔc±μ ΨΘ©Θ§ Β―ιa¥”Ζ¥”ΠΩΣ Φ÷Ν45s¥οΒΫΤΫΚβΘ§‘ρΗΟΙΐ≥ΧΖ¥”ΠΥΌ¬ vΘ®SO2Θ©__________Θ®ΫαΙϊ±ΘΝτ2ΈΜ”––ß ΐΉ÷Θ©ΓΘ
SΘ®sΘ©+2CO2Θ®gΘ© H = -270 kJΓΛmolΘ≠1Θ§Ζ¥”ΠΧεœΒΉή―ΙΥφ ±ΦδΒΡ±δΜ·»γœ¬±μΥυ ΨΘ§‘ρ»ΐΉι Β―ιΈ¬Ε»ΒΡ¥σ–ΓΙΊœΒ «_____Θ®”ΟaΓΔbΓΔc±μ ΨΘ©Θ§ Β―ιa¥”Ζ¥”ΠΩΣ Φ÷Ν45s¥οΒΫΤΫΚβΘ§‘ρΗΟΙΐ≥ΧΖ¥”ΠΥΌ¬ vΘ®SO2Θ©__________Θ®ΫαΙϊ±ΘΝτ2ΈΜ”––ß ΐΉ÷Θ©ΓΘ
| 0s | 40s | 45s | 60s |
a | 175 | 142 | 140 | 140 |
b | 160 | 120 | 120 | 120 |
c | 160 | 130 | 125 | 120 |
Θ®2Θ©άϊ”ΟCOΓΔH2Ω…÷Τ±ΗΧλ»ΜΤχΘ§÷ς“ΣΖ¥”ΠΈΣΘΚ
COΘ®gΘ© + 3H2Θ®gΘ© ![]() CH4Θ®gΘ© + H2OΘ®gΘ© H1=-206.2 kJΓΛmol1ΘΜ
CH4Θ®gΘ© + H2OΘ®gΘ© H1=-206.2 kJΓΛmol1ΘΜ
COΘ®gΘ© + H2OΘ®gΘ© ![]() CO2Θ®gΘ© + H2Θ®gΘ© H2 = -41.0 kJΓΛmolΘ≠1ΘΜ
CO2Θ®gΘ© + H2Θ®gΘ© H2 = -41.0 kJΓΛmolΘ≠1ΘΜ
H2OΘ®lΘ© ®TH2OΘ®gΘ© H3 =+44 kJΓΛmolΘ≠1 ΓΘ
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
ΔΌΖ¥”ΠCO2Θ®gΘ© + 4H2Θ®gΘ© ![]() CH4Θ®gΘ© + 2H2OΘ®lΘ© ΒΡH4 = ________ kJΓΛmolΘ≠1ΓΘΡ≥Έ¬Ε»œ¬Θ§Ζ÷±π‘ΎΤπ Φ»ίΜΐœύΆ§ΒΡΚψ―Ι»ίΤςAΓΔΚψ»ί»ίΤςB÷–Φ”»Υ1molCO2ΚΆ4molH2ΒΡΜλΚœΤχΧεΘ§ΝΫ»ίΤςΖ¥”Π¥οΒΫΤΫΚβΚσΖ≈≥ωΜρΈϋ ’ΒΡ»»ΝΩΫœΕύΒΡ «__Θ® ΧνΓΑAΓ±ΜρΓΑB"Θ©ΓΘ
CH4Θ®gΘ© + 2H2OΘ®lΘ© ΒΡH4 = ________ kJΓΛmolΘ≠1ΓΘΡ≥Έ¬Ε»œ¬Θ§Ζ÷±π‘ΎΤπ Φ»ίΜΐœύΆ§ΒΡΚψ―Ι»ίΤςAΓΔΚψ»ί»ίΤςB÷–Φ”»Υ1molCO2ΚΆ4molH2ΒΡΜλΚœΤχΧεΘ§ΝΫ»ίΤςΖ¥”Π¥οΒΫΤΫΚβΚσΖ≈≥ωΜρΈϋ ’ΒΡ»»ΝΩΫœΕύΒΡ «__Θ® ΧνΓΑAΓ±ΜρΓΑB"Θ©ΓΘ
ΔΎ‘ΎΚψ―ΙΙήΒάΖ¥”ΠΤς÷–Α¥nΘ®H2Θ©:nΘ®COΘ© = 3:1Ά®»κ‘≠ΝœΤχΘ§‘Ύ¥ΏΜ·ΦΝΉς”Οœ¬÷Τ±ΗΚœ≥…Χλ»ΜΤχΘ§400 Γφ pΉήΈΣ100 kPa ±Ζ¥”ΠΧεœΒΤΫΚβΉι≥…»γœ¬±μΥυ ΨΘΚ
ΉιΖ÷ | CH4 | H2O | H2 | CO2 | CO |
ΧεΜΐΖ÷ ΐ/% | 45.0 | 42.5 | 10.0 | 1.50 | 1.00 |
‘ρΗΟΧθΦΰœ¬COΒΡΉήΉΣΜ·¬ ΠΝ=____ΓΘ![]()
Δέ÷Τ±ΗΚœ≥…Χλ»ΜΤχ≤…”Ο‘Ύ‘≠ΝœΤχ÷–Ά®»κΥ°’τΤχά¥ΜΚΫβ¥ΏΜ·ΦΝΜΐΧΦΓΘ
ΜΐΧΦΖ¥”ΠΈΣΘΚΖ¥”ΠI ΘΚCH4Θ®gΘ© ![]() CΘ®sΘ© + 2H2Θ®gΘ© H = +75 kJΓΛmolΘ≠1ΘΜ
CΘ®sΘ© + 2H2Θ®gΘ© H = +75 kJΓΛmolΘ≠1ΘΜ
Ζ¥”ΠΔρΘΚ2COΘ®gΘ© ![]() CΘ®sΘ© + CO2Θ®gΘ© H = -172 kJΓΛmolΘ≠1Θ§
CΘ®sΘ© + CO2Θ®gΘ© H = -172 kJΓΛmolΘ≠1Θ§
ΤΫΚβΧεœΒ÷–Υ°’τΤχ≈®Ε»Ε‘ΜΐΧΦΝΩΒΡ”Αœλ»γΆΦΥυ ΨΘ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «__ΓΘ
A «ζœΏ1‘Ύ700 ~ 800ΓφΜΐΧΦΝΩΦθ–ΓΒΡ‘≠“ρΩ…Ρή «Ζ¥”ΠΔρΡφœρ“ΤΕ·
B «ζœΏ1‘Ύ550 ~700ΓφΜΐΧΦΝΩ‘ω¥σΒΡ‘≠“ρΩ…Ρή «Ζ¥”ΠIΓΔΔρΒΡΥΌ¬ ‘ω¥σ
C «ζœΏ2ΓΔ3‘Ύ550 ~800ΓφΜΐΧΦΝΩΫœΒΆΒΡ‘≠“ρ «Υ°’τΤχœΓ ΆΉς”Ο ΙΜΐΧΦΖ¥”ΠΥΌ¬ Φθ–Γ
D Υ°’τΤχΡήΈϋ ’Ζ¥”ΠΖ≈≥ωΒΡ»»ΝΩΘ§ΫΒΒΆΧεœΒΈ¬Ε»÷Ν550Γφ“‘œ¬Θ§”–άϊ”ΎΦθ…ΌΜΐΧΦ

≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ»ΦΟΚΆ―ΝρΒΡΖ¥”ΠΈΣ2CaCO3(s)+2SO2(g)+O2(g)=2CaSO4(s)+2CO2(g) ΓςH<0ΓΘ500Γφ ±Θ§ Β―ι≤βΒΟ‘Ύ2LΗ’–‘Οή±’»ίΤς÷–ΗΟΖ¥”Π‘Ύ≤ΜΆ§ ±ΩΧO2ΓΔCO2ΒΡΈο÷ ΒΡΝΩΘ®ΜρΈο÷ ΒΡΝΩ≈®Ε»Θ©»γœ¬±μΥυ ΨΘ§œ¬Ν–≈–Εœ’ΐ»ΖΒΡ «Θ® Θ©
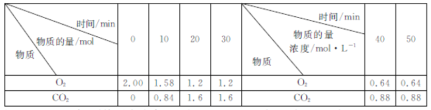
A.0ΓΪ10minΡΎΘ§ΤΫΨυΖ¥”ΠΥΌ¬ v(SO2)=0.084molΓΛL-1ΓΛmin-1
B.30minΚσΘ§ΗΡ±δΒΡΧθΦΰ÷ΜΡή «‘ω¥σO2ΒΡΆ®»κΝΩ
C.»τ»ίΤςΨχ»»Θ§‘ρ20min ±Θ§![]() ΘΨ
ΘΨ![]()
D.Μ·―ßΤΫΚβ≥Θ ΐΘΚ30min<40min
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΜ·ΚœΈοH «C5a ήΧεόΉΩΙΦΝW-54011ΒΡ÷–ΦδΧεΘ§NakamuraΒ»»Υ…ηΦΤ÷Τ±ΗHΒΡΚœ≥…¬ΖœΏ»γΆΦΥυ ΨΘΚ
“―÷ΣΘΚΔώ. ΘΜ
ΘΜ
Δρ.R-CN![]() R-COOHΓΘ
R-COOHΓΘ
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©B÷–Κ§―θΙΌΡήΆ≈ΒΡΟϊ≥ΤΈΣ___ΓΘ
Θ®2Θ©FΒΡΖ÷Ή” ΫΈΣC13H13OClΘ§‘ρFΒΡΫαΙΙΦρ ΫΈΣ___ΓΘ
Θ®3Θ©ΔΎΔίΒΡΖ¥”Πάύ–ΆΖ÷±πΈΣ___ΓΔ___ΓΘ
Θ®4Θ©Ζ¥”ΠΔΌΒΡΜ·―ßΖΫ≥Χ ΫΈΣ___ΓΘ
Θ®5Θ©ΧΦ‘≠Ή”…œΝ§”–4Ηω≤ΜΆ§ΒΡ‘≠Ή”ΜρΜυΆ≈ ±Θ§ΗΟΧΦ≥ΤΈΣ ÷–‘ΧΦΘ§–¥≥ωΜ·ΚœΈοH”κΉψΝΩH2ΖΔ…ζΦ”≥…Ζ¥”ΠΒΡ≤ζΈοΒΡΫαΙΙΦρ Ϋ___Θ§≤Δ”Ο–«Κ≈(*)±ξ≥ωΤδ÷–ΒΡ ÷–‘ΧΦΘΚ
Θ®6Θ©Q”κCΜΞΈΣΆ§Ζ÷“λΙΙΧεΘ§Q”ωFeCl3»ή“ΚΖΔ…ζœ‘…ΪΖ¥”ΠΘ§«“±ΫΜΖ…œΝ§”–ΝΫΗω»Γ¥ζΜυΘ§1molQ”κΉψΝΩNaHCO3»ή“ΚΖ¥”ΠΉνΕύ≤ζ…ζ1molCO2Θ§‘ρQΒΡΆ§Ζ÷“λΙΙΧε”–___÷÷Θ®≤ΜΚ§ΝΔΧε“λΙΙΘ©Τδ÷–ΚΥ¥≈Ι≤’ώ«βΤΉ”–6ΉιΈϋ ’ΖεΒΡΫαΙΙΦρ ΫΈΣ___(»Έ–¥“Μ÷÷)ΓΘ
Θ®7Θ©≤Έ’’…œ ωΚœ≥…¬ΖœΏΚΆ–≈œΔΘ§…ηΦΤ“‘±Ϋ““»©ΈΣ‘≠Νœ(ΤδΥϋ ‘ΦΝ»Έ―Γ)Θ§÷Τ±Η![]() ΒΡΚœ≥…¬ΖœΏΘΚ___ΓΘ
ΒΡΚœ≥…¬ΖœΏΘΚ___ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–”–ΙΊ Β―ιΖΫΖ®ΚΆΫα¬έΕΦ’ΐ»ΖΒΡ «
A.ΥαΦν÷–ΚΆΒΈΕ® ±Θ§ΉΕ–ΈΤΩ–η”Ο¥ΐ≤β“Κ»σœ¥2¥ΈΘ§‘ΌΦ”»κ¥ΐ≤β“Κ
B.Φλ―ι’αΧ«Υ°Ϋβ≤ζΈοΨΏ”–ΜΙ‘≠–‘ΘΚ‘Ύ’αΧ«Υ°ΫβΚσΒΡ»ή“Κ÷–œ»Φ”»κ ΝΩœΓNaOH»ή“Κ÷–ΚΆΘ§‘ΌΦ”»κ–¬÷ΤΒΡ“χΑ±»ή“Κ≤ΔΥ°‘ΓΦ”»»
C.ΆΦ÷–Υα ΫΒΈΕ®ΙήΕΝ ΐΈΣ12.20 mL
D.»τ”Ο“―÷Σ≈®Ε»ΒΡKMnO4»ή“Κ»Ξ≤βΕ®Ρ≥»ή“Κ÷–Fe2+≈®Ε»Θ§KMnO4»ή“ΚΖ≈‘ΎΦν ΫΒΈΕ®Ιή÷–
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΫϋΡξά¥Θ§ΙΎ“‘ΓΑ¬Χ…ΪΓ±ΒΡ–¬Η≈Ρν≤ΜΕœ≤ζ…ζΘ§»γ¬Χ…Ϊ ≥ΤΖΓΔ¬Χ…Ϊ≤ΡΝœΓΔ¬Χ…ΪΡή‘¥ΓΔ¬Χ…ΪΜ·―ßΒ»Θ§’βάοΒΡΓΑ¬Χ…ΪΓ± «Ε‘»Υάύ…γΜαΩ…≥÷–χΖΔ’Ι’Ϋ¬‘ΒΡ–Έœσ±μ ωΓΘΓΑ¬Χ…ΪΜ·―ßΓ±“Σ«σ¥”Ψ≠ΦΟΓΔΜΖ±ΘΚΆΦΦ θ…œ…ηΦΤΩ…––ΒΡΜ·―ßΖ¥”ΠΓΘΨί¥ΥΘ§”…ΒΞ÷ ΟΨ÷ΤœθΥαΟΨΒΡœ¬Ν–4ΗωΖΫΑΗ÷–Θ§Ρψ»œΈΣΩ…––Εχ«“ΖϊΚœΓΑ¬Χ…ΪΜ·―ßΓ±“Σ«σΒΡΖΫΑΗ «Θ® Θ©
A.![]()
B.![]()
C.Mg![]() MgO
MgO![]() Mg(NO3)2
Mg(NO3)2
D.![]()
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ “Έ¬œ¬Θ§œρ10 mL 0.1 mol/L¥ΉΥα»ή“Κ÷–Φ”Υ°œΓ ΆΚσΘ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ® Θ©
A.»ή“Κ÷–ΝΘΉ”ΒΡ ΐΡΩΦθ–Γ
B.‘ΌΦ”»κCH3COONaΙΧΧεΡή¥ΌΫχ¥ΉΥαΒΡΒγάκ
C.¥ΉΥαΒΡΒγάκ≥ΧΕ»‘ω¥σΘ§c(HΘΪ)“ύ‘ω¥σ
D.»ή“Κ÷–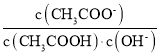 ≤Μ±δ
≤Μ±δ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com