
����Ŀ���������ƣ�Na2FeO4���Ǿ�����ɫ����ķ�ĩ����һ�ָ�Ч��ɫǿ�������������������ȶ��������ڷ�ˮ��������ˮ�Ĵ�����ʵ������ʯī������Ϊ�缫���Բ�ͬŨ�ȵ�NaOH��ҺΪ�������Һ������һ����ѹ����Ʊ��������ƣ����װ�ú�����������
c(NaOH) | �������� | �������� |
1 mol��L��1 | ������ɫ���� | ������ɫ���壬10min����Һ��ɫ�����Ա仯 |
10 mol��L��1 | ����������ɫ���� | ����������ɫ���壬3min����Һ��Ϊdz�Ϻ�ɫ��������� |
15 mol��L��1 | ����������ɫ���� | ����������ɫ���壬1min����Һ��Ϊdz�Ϻ�ɫ��������� |
����˵������ȷ����

A. aΪ������bΪʯī
B. ������Ҫ������Ӧ��2H2O + 2e��=== H2��+ 2OH��
C. ��Ũ�ȵ�NaOH��Һ�������ڷ���Fe��6e��+ 8OH��=== FeO42��+ 4H2O
D. �Ʊ�Na2FeO4ʱ�����ñ���NaCl��Һ������Ч����������������
���𰸡�D
�������������������������Ϣ��֪������Ϊ������ʯīΪ������������ˮ����������ӷŵ�����������������������Һ��Ũ�Ⱥܴ�ʱ�������ϼ������������ӷŵ�����������������������ΪFeO42����A. aΪ������bΪʯī��A��ȷ��B. ������Ҫ�����ĵ缫��Ӧ��2H2O + 2e��=== H2��+ 2OH����B��ȷ��C. ��Ũ�ȵ�NaOH��Һ�������ڷ���Fe��6e��+ 8OH��=== FeO42��+ 4H2O��C��ȷ��D. �Ʊ�Na2FeO4ʱ�����ñ���NaCl��Һ�������������ӷŵ����������D����ȷ������ѡD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ����12mol��L��1��Ũ��������0.10mol��L��1��ϡ����500 mL���ش��������⡣
��1����������ƿ��ʹ�ã����в�����ȷ����___(�����)��
a.ʹ��ǰҪ��������ƿ�Ƿ�©Һ
b.������ˮϴ�Ӻ����Ҫ������ƿ���
c.Ϊ�˱��ڲ�����Ũ��Һϡ�ͻ�����ܽ��ֱ��������ƿ�н���
d.Ϊ��ʹ������ҺŨ�Ⱦ��ȣ����ݽ�����Ҫҡ��
e.��500mL������ƿ����ֱ������480mL��Һ
f.��������ƿ��������Һ��������ƿ������Ƶ��Լ�
��2����ȡŨ��������Ϊ___mL��Ӧѡ�õ���Ͳ���Ϊ___(����10mL����25mL������50mL��)��
��3������ʱӦѡ�õ�����ƿ���Ϊ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������һ�ֳ��õĻ��ʣ��乤ҵ����������ͼ����ش��������⡣
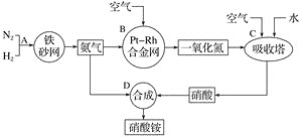
��1��д����Ӧ����B�з�����Ӧ�Ļ�ѧ����ʽ��____��
��2��������C��ͨ�������Ŀ����___��C��D������Ӧ�����з����ķ�Ӧ������������ԭ��Ӧ����___(�Ӧ��������)��
��3��Ũ����һ�㱣������ɫ�Լ�ƿ������������������û�ѧ����ʽ����ԭ��___��
��4��̼��Ũ���ᷴӦ�Ļ�ѧ����ʽ��_____��
��5����128gͭ����һ������Ũ�����в��ȡ���ͭƬ��ȫ��ʧʱ�����ռ���NO2��NO�Ļ������44.8L(��״��)��
��д�����������У��йط�Ӧ�����ӷ���ʽ��____��____��
����ɸ÷�Ӧ������Ҫ��ȡ10mol��L��1��Ũ����_____mL��
�ۻ��������NO2�����Ϊ____L��NO�����Ϊ____L(��Ϊ��״����)��
�ܽ��ռ��������������������ʢ��ˮ��ˮ���У��������л���ͨ��O2ʹ���ַ�Ӧ����Ҫʹ��Һǡ�ó�������������������Ҫ�μӷ�Ӧ��O2�����ʵ���Ϊ____mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��֤����ʵ�����Ʊ��õ���Cl2�л����HCl����ͬѧ�������ͼ��ʾ��ʵ��װ�ã���Ҫ��ش��������⡣
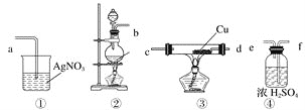
��1������ݼ�ͬѧ��ʾ��ͼ������ʵ��װ�ô������ҵ�����˳��װ�â���__________��
��2��װ�â�����Ҫ��������������__________��__________��__________��
��3��ʵ�����Ʊ�Cl2�����ӷ���ʽΪ____________��
��4��װ�â���Cu������__________���û�ѧ����ʽ��ʾ����
��5����ͬѧ��Ϊ��ͬѧʵ�������Ȼ����ȱ�ݣ�����֤������ͨ��AgNO3��Һ�е�����ֻ��һ�֡�Ϊ�ˣ���ͬѧ�������ͨ��װ�â�֮ǰ��Ҫ��װһ������װ�âݣ���֤������ͨ��AgNO3��Һ�е�����ֻ��һ�֡�����Ϊװ�â�Ӧ����__________��
��6����ͬѧ������ͬѧ��Ƶ�װ�ú����������װ�ã�ֻ�轫ԭ���ձ��е�AgNO3��Һ������ɫʯ����Һ������۲쵽__________��������֤����Cl2ʱ��HCl�ӷ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������CH4������NO2����Ⱦ����Ӧԭ��Ϊ��CH4(g)+2NO2(g) N2(g) + CO2(g) +2H2O(g)����10L�ܱ������зֱ����0.50mol CH4 ��1.2mol NO2����ò�ͬ�¶��� n(CH4)��ʱ��仯���й�ʵ�����������ʾ������˵����ȷ����
��� | �¶�/K | ʱ��/min ���ʵ���/mol | 0 | 10 | 20 | 40 | 50 |
�� | T1 | n(CH4) | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
�� | T2 | n(CH4) | 0.50 | 0.30 | 0.18 | M | 0.15 |
A.��ʵ�����ݿ�֪�¶� T 1��T2
B.������ 0 ~20 min �ڣ�NO2 ��������Ϊ0.0125 molL-1min-1
C.40 min ʱ�������� M ��Ӧ������Ϊ 0.18
D.�÷�Ӧֻ���ڸ����²����Է�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ����������ij�ܱ������м��� 0.3 mol A��0.1 mol C ��һ������ B �������壬ͼ 1 ��ʾ������Ũ����ʱ��ı仯��ͼ 2 ��ʾ������ʱ��ı仯��t2��t3��t4��t5 ʱ�̸��ı�һ���������Ҹı����������ͬ����t4ʱ�� �ı��������ѹǿ��������˵���������

A.�� t1=15 s����ǰ 15 s ��ƽ����Ӧ���� v(C)=0.004 mol��L-1��s-1
B.�÷�Ӧ�Ļ�ѧ����ʽΪ 3A(g)![]() B(g)+2C(g)
B(g)+2C(g)
C.t2��t3��t5 ʱ�̸ı�������ֱ��������¶ȡ��������������Ӧ��Ũ��
D.�� t1=15 s���� B ����ʼ���ʵ���Ϊ 0.04 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����к͵ζ����ⶨ�ռ�Ĵ��ȣ����ռ��к����������������õĿ��������ʣ��Ը���ʵ��ش�
��1��ȷ��ȡ4.0g�ռ���Ʒ��
��2������Ʒ���250mL����Һ��
��3����____________�����������ƣ���ȡ25.00mL����Һ����ƿ�У����μӼ��μ�����ָʾ����
��4����0.2010 mol��L����������ζ������ռ���Һ���ζ�ʱ������ע��____________��ֱ���ζ��յ㡣�ﵽ�յ�ľ��������ǣ�____________��
��5��������ʵ��ζ����������±���
�ζ����� | ����Һ�����mL�� | �����������mL�� | |
�ζ�ǰ������mL�� | �ζ��������mL�� | ||
��һ�� | 25.00 | 0.50 | 20.40 |
�ڶ��� | 25.00 | 5.00 | 28.30 |
������ | 25.00 | 4.00 | 24.10 |
�������������ݣ������ռ�Ĵ��ȣ�____________
��6�����в����У��ᵼ������õ��ռ�Ĵ���ƫ�����________��
a���ζ��յ�ʱ�����ӿ̶�
b��û�����������Һ��ϴ��Ӧ�ĵζ���
c����ƿ��������������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ͪ�������л��ϳ��Լ���ָʾ������ṹ��ͼ��ʾ������˵��������ǣ� ��

A.���ͪ�ķ���ʽΪC15H12O
B.���ͪ���������е�̼ԭ�ӿ��ܹ�ƽ��
C.���ͪ�ܷ����Ӿ۷�Ӧ�Ʊ��߷���
D.���ͪ��ͬ���칹���к���̼��˫����̼̼˫������������ֱ�������Ĺ���6��(�����������칹)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��һ����ʵ��Ϊ������ѧ�ƣ�ʵ��̽���ܼ���ѧ��ѧϰ��ѧ����Ȥ��ij��ѧ��ȤС�������ͼʵ��װ�ã��г��豸���ԣ��Ʊ�������̽����������±��Ԫ�ص����ʡ��ش��������⣺

(1)����a��������______________��
(2)Aװ���з����Ļ�ѧ��Ӧ����ʽΪ_________________________________������Ư�ۻ���KClO3����Ӧ��ÿ����21.3g Cl2ʱת�Ƶĵ�����ĿΪ____NA��
(3)װ��B�����ڼ��ʵ�������C���Ƿ��������C�������˶�������B�пɹ۲쵽__________��
(4)װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���ʱC�Т������������οɷ���____����ѡ��a��b��c����
ѡ�� | �� | �� | �� |
a | �������ɫ���� | Ũ���� | ʪ�����ɫ���� |
b | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
c | ʪ�����ɫ���� | ��ʯ�� | �������ɫ���� |
(5)���װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ����������ʱ���ɹ۲쵽��ɫ��Һ��Ϊ����ɫ��˵���ȵķǽ����Դ����壬��������D��������Һ����E�У���E���۲쵽��������_______________________________��������_____����������������������˵����ķǽ�����ǿ�ڵ⣬ԭ����_____________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com