
����Ŀ������˵����ȷ����
A. ����ʱ��0.1mol��L-1��������Һ��NH4Al(SO4)2 ��NH4Cl ��NH3��H2O ��CH3COONH4 �У�c (NH4+)�ɴ�С��˳���ǣ���>��>��>��
B. 0.1mol��L-1 NaHCO3��Һ�У�c(Na+)= 2c(CO32-)+ c(HCO3-)+c(H2CO3)
C. ����ʱ��pH=2��CH3COOH��Һ��HCl��Һ��pH=12�İ�ˮ��NaOH��Һ��������Һ����ˮ�����c(H+)�����
D. ����ʱ��0.3 mol��L-1HY��Һ��0.3 mol��L-1NaOH��Һ�������Ϻ���Һ��pH=9��������Һ��c(H+) +c(HY)= 1��10-5 mol��L-1
���𰸡�D
��������ͬŨ�ȵ�������Һ: ��NH4Al(SO4)2 ��NH4Cl ��NH3��H2O ��CH3COONH4 ,��(1)��������ˮ������笠����ӵ�ˮ��; (2)��笠�����ˮ��; (3)���������,�ҵ���ij̶Ⱥ���; (4)���������ˮ��ٽ�笠�����ˮ��,��c (NH4+)�ɴ�С��˳����: (1) >(2)> (4)> (3) ����A�Ǵ����; B. ���������غ�0.1mol��L-1 NaHCO3��Һ�У�c(Na+)= c(CO32-)+ c(HCO3-)+c(H2CO3)����B��������ʱ��pH=2��CH3COOH��Һ��HCl��Һ�У�ˮ�����������Ϊ10-12mol/L��pH=12�İ�ˮ��NaOH��Һ�У�ˮ�����������Ϊ10-12mol/L,����������Һ����ˮ�����c(H+)��ȣ���C����D. 0.3 mol/L HY��Һ��0��3 mol/L NaOH��Һ�������Ϻ�,�õ�����Һ��NaY��Һ,��֪pH = 9,˵��HY������,����NaY������ˮ��.����Һ�д��������غ㣺c(![]() ) = c(
) = c(![]() ) + c(HY)��Һ��ͬʱ���ڵ���غ㣺c(
) + c(HY)��Һ��ͬʱ���ڵ���غ㣺c(![]() ) + c(
) + c(![]() ) = c(
) = c(![]() ) + c(
) + c(![]() )
)
��1ʽ- 2ʽ�ã�c(![]() ) - c(
) - c(![]() ) = c(
) = c(![]() )��ΪpH= 9, c(
)��ΪpH= 9, c(![]() ) = 10-9mol/L����c(H+) +c(HY)= 1��10-5 mol��L-1����D��ȷ���𰸣�D��
) = 10-9mol/L����c(H+) +c(HY)= 1��10-5 mol��L-1����D��ȷ���𰸣�D��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й������������仯���ǣ� ��
A.��¯����B.��ˮɹ��C.���Ⱥ���D.�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������һ�������˿ڵĽ�����衣���������ijɷ��ж��֣����ᱽ����![]() �����е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɡ�һ�ֺϳ�·�����£�
�����е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɡ�һ�ֺϳ�·�����£�
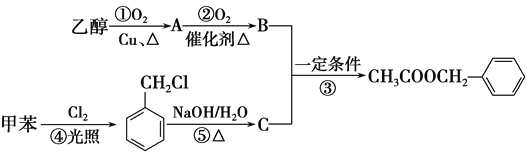
(1)B��C�Ľṹ��ʽ�ֱ�Ϊ______________��________________��
(2)д����Ӧ�١��ܵĻ�ѧ����ʽ��
��_____________________����______________________��
(3)��Ӧ�ۡ��ݵķ�Ӧ���ͷֱ�Ϊ��________����____________��
(4)��Ӧ________(�����)ԭ�ӵ�����������Ϊ100%�����ϡ���ɫ��ѧ����Ҫ��
(5)���ᱽ������ͬ���칹��������̼�����Ʒ�Ӧ�����������________�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. �κ������£���ѧ��Ӧ���ʱ䶼���ڻ�ѧ��Ӧ�ķ�Ӧ��
B. ��Ҫ���Ȳ��ܽ��еķ�Ӧһ�������ȷ�Ӧ
C. ���ʾ��е�����Խ�ߣ����ʵ��ȶ���Խǿ
D. ��ȷ���Ļ�ѧ��Ӧ��ϵ�У���Ӧ������������������������һ����ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�Ҷ�һ̼��ѧ�����˹㷺������о���ȡ����һЩ��Ҫ�ɹ���
��1����֪��CO(g)+2H2(g)![]() CH3OH(g) ��H1=-90.1kJ��mol-1��
CH3OH(g) ��H1=-90.1kJ��mol-1��
3CH3OH(g)![]() CH3CH=CH2(g)+3H2O(g) ��H2=-31.0 kJ��mol-1��
CH3CH=CH2(g)+3H2O(g) ��H2=-31.0 kJ��mol-1��
CO��H2�ϳ�CH3CH=CH2���Ȼ�ѧ����ʽΪ______________________________________��
��2���������������Ϊ2L�ĺ����ܱ��������������ֱ����1molCO��2molH2��������Ӧ��CO(g)+2H2(g)![]() CH3OH(g) ��H=-90.1kJ��mol-1�����������ķ�Ӧ�¶ȷֱ�ΪT1��T2��T3�Һ㶨���䡣����Ӧ�����е�5minʱH2�����������ͼ1��ʾ������ֻ��һ�������еķ�Ӧ�Ѿ��ﵽƽ��״̬��
CH3OH(g) ��H=-90.1kJ��mol-1�����������ķ�Ӧ�¶ȷֱ�ΪT1��T2��T3�Һ㶨���䡣����Ӧ�����е�5minʱH2�����������ͼ1��ʾ������ֻ��һ�������еķ�Ӧ�Ѿ��ﵽƽ��״̬��
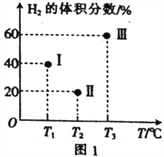
��5minʱ���������еķ�Ӧ�ﵽ��ѧƽ��״̬��������_____________�����������
��0~5min������������CH3OH��ʾ�Ļ�ѧ��Ӧ����v(CH3OH)=_________________��
�������������еķ�Ӧ���ﵽƽ��״̬ʱ��CO��ת������ߵ�������____________�����������ͬ����ƽ�ⳣ����С��������____________________��
��3��CO�����ڹ�ҵұ���������ڲ�ͬ�¶�����CO��ԭ���ֽ������������ﵽƽ���������lg![]() ���¶���T���Ĺ�ϵ��ͼ2��ʾ������˵����ȷ����_____________������ĸ����
���¶���T���Ĺ�ϵ��ͼ2��ʾ������˵����ȷ����_____________������ĸ����
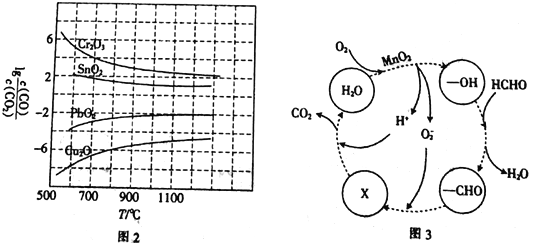
a.��ҵ�Ͽ���ͨ�����߷�Ӧװ�����ӳ���ʯ��CO�Ӵ���ʱ�䣬����β����CO�ĺ���
b.CO���ڹ�ҵұ��������(Cr)ʱ����ԭЧ�ʲ���
c.��ҵұ������ͭ(Cu)ʱ��600����CO�������ʱ�100����CO�������ʸ���
d.CO��ԭPbO2��Ӧ����H>0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾʵ��װ���У���ʢ����Һ�ٺ���Һ�ڵ��Թ��У�ͨ����������X��������Һ�١���Һ�ھ��������ɵ�����

ѡ�� | ����X | ��Һ�� | ��Һ�� |
A | SO2 | Ca(OH)2 | BaCl2 |
B | Cl2 | AgNO3 | Na2S |
C | NH3 | AgNO3 | AlCl3 |
D | HCl | Na2SiO3 | NaAlO2 |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�����������˵�й�˵����ȷ����
A. �ڳ��³�ѹ�£�11.2L N2���еķ�����С��0.5NA
B. ��״���£�2.24L SO3���е�ԭ����Ϊ0.4NA
C. ��1L 2mol/L��FeCl3��Һ�����к���Cl-Ϊ2NA
D. 46g NO2��N2O4�������������ԭ����Ŀ�п���Ϊ3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ϩ�����廯�⡢ˮ�ӳ�ʱ������������֮�֣����磺
![]()

���п�ͼ��B��C��D������ط�Ӧ�е���Ҫ����(�����������Լ���ʡ��)���һ�����B�н���4��̼ԭ�ӡ�1����ԭ�ӡ�1����ԭ�ӡ�

������ͼ�У�B�Ľṹ��ʽΪ________������ȡ����Ӧ����__________(���ͼ�����)��������ȥ��Ӧ����__________(�����)��д����Ӧ�ܵĻ�ѧ����ʽ(ֻд��Ҫ���������Ӧ����)��__________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijУѧ��С����������ͼװ�úϳ����������IJ������£���Բ����ƿA �ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װ����B�����Ȼ���һ��ʱ�������װ�ý������õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺

��1������B������Ϊ_____������ˮ�����뷽����_______________���a����b������
��2������ƿ�з������Ƭ����������___________________���������һ��ʱ����������������Ƭ��Ӧ�ò�ȡ����ȷ������___________������ĸ����
A.��ȴ�� B.�������� C.���貹�� D.�������Ϻ����
��3����Ӧ�м�������Ҵ�����Ŀ����___________________����������Ũ���ἴ��������ã���ʵ���������ڴ�����ԭ����______________��Ũ���������ֲ��ܹ��࣬ԭ����________________________��
��4�����������ʵ�鲽���Ϊ��������ƿ���ȼ����Ҵ���Ũ���ᣬȻ��ͨ����Һ©���ߵμ����ᣬ��������������������������IJ��ʣ���ԭ����__________________________________��
��5���ס�����λͬѧ�������ú����Ҵ��������ˮ�����������ֲ�Ʒ�ᴿ�����Ƿֱֲ�Ʒ��һ����NaOH��Һ��Ϻ������ռ�76��~78��IJ�Ʒ(��֪���Ҵ��ķе�78�棬����ķе�117.9�棬���������ķе�77��)��ʵ�������õ�����ˮ��Һ��X���ҵõ�������ˮ���������Ե�Һ��Y����X��Ҫ��_________________________����Y��Ҫ_________________________��__________________________��
��6����ͬѧ����������ᴿ�ֲ�Ʒ�ķ������������£�

�ɴ˻ش��������⣺
���Լ�a�ǣ�_______________ �����������ܽ��Ҵ�����Ӧ���������____________________��
�ڷ��뷽��[��]��_____________���Լ�b��___________�����뷽��[��]��_____________��
��7����30g������46g�Ҵ���Ӧ������Ϊ67%�����Ƶõ���������������Ϊ__________g������3λ��Ч���֣���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com