
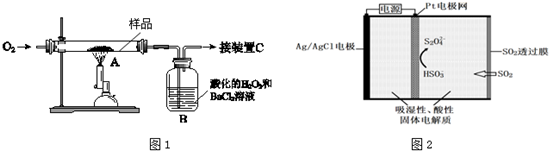
���� ��1����ȡm g����ҩ��Ʒ��װ��A�У�����ַ�Ӧ��FeS2ȼ�����ɶ������������������������ͨ���ữ�Ĺ���������Ȼ�����Һ�У�ʹ��Ԫ��ȫ��ת��ΪSO2��SO3����B�еõ���ɫ����������������ԭ��Ӧ�������ᱵ������
��Ԫ���غ��������Ҫ�ⶨB�г�������������Ԫ�����ʵ�����
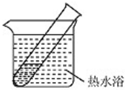
������������ԭ��Ӧ������Һ��������������ӵõ���������S2O42-��
��2���ٽ�Aװ���е�ʣ��������������������������H2��������ַ�Ӧ����ˣ��õ���ɫ��Һ��˵������������������
������Һ�еμ�TiCl3��Һ����ǡ����ȫ��Ӧ��TiCl3������������ΪTiO2+��
���õζ����ⶨFe2+����������v mL n mol/LK2Cr2O7��Һ�����ݻ�ѧ��Ӧ�Ķ�����ϵ����õ���
��� �⣺��1����ȡm g����ҩ��Ʒ��װ��A�У�����ַ�Ӧ��FeS2ȼ�����ɶ������������������������ͨ���ữ�Ĺ���������Ȼ�����Һ�У�ʹ��Ԫ��ȫ��ת��ΪSO2��SO3����B�еõ���ɫ����������������ԭ��Ӧ�������ᱵ�����������������Ҫ����������������+4����Ϊ+6����������ӣ�װ��B�е�H2O2��Ӧʱ���ֳ��������ԣ�
�ʴ�Ϊ��������
����������Ԫ�ص�������������Ҫ�ⶨB�г�������������Ԫ�����ʵ��������Բ�����������B�г�����������
�ʴ�Ϊ��B�г�����������
������������ԭ��Ӧ������Һ��������������ӵõ���������S2O42-���缫��ӦΪ2HSO3-+2H++2e-=S2O42-+2H2O��
�ʴ�Ϊ��2HSO3-+2H++2e-=S2O42-+2H2O��
��2���ٽ�Aװ���е�ʣ��������������������������H2����֤������������ַ�Ӧ����ˣ��õ���ɫ��Һ��˵�����������������������ܽ��õ���Һ�к���������Ϊ��Fe3+��Fe2+��
�ʴ�Ϊ��Fe3+��Fe2+��
������Һ�еμ�TiCl3��Һ����ǡ����ȫ��Ӧ��TiCl3������������ΪTiO2+����Ӧ�����ӷ���ʽΪ��Fe3++Ti3++H2O=Fe2++TiO2++2H+��
�ʴ�Ϊ��Fe3++Ti3++H2O=Fe2++TiO2++2H+��
���õζ����ⶨFe2+����������v mL n mol/LK2Cr2O7��Һ����
6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O
6 1
n��Fe2+�� v��10-3L��n mol/L
n=6vn��10-3mol
��Ʒ����Ԫ����������=$\frac{6vn��1{0}^{-3}mol��56g/mol}{mg}$��100%=$\frac{0.336}{m}$vn��100%=$\frac{0.336vn}{m}$��100%=$\frac{33.6nv}{m}%$��
�ʴ�Ϊ��$\frac{33.6nv}{m}%$��
���� ���⿼����������ɵķ����жϣ���Ҫ��ʵ�鹤����������Ӧʵ�ʵ�����Ӧ�ã��������ʺͷ�Ӧ���������Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�


 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol•L-1��NaHS��Һ������Ũ�ȹ�ϵ��c��OH-��=c��H+��-c��S2-��+c��H2S�� | |
| B�� | Ũ��Ϊ0.1mol•L-1��̼������Һ��c��Na+��=2c��CO32-��+c��HCO3-��+c��H2CO3�� | |
| C�� | pH=12�İ�ˮ��pH=2������������ϣ�c��Cl-����c��NH4+����c��H+����c��OH-�� | |
| D�� | ������Һ��NaOH��Һ���Ϻ�������Һ�����ԣ�c��Na+����c��CH3COO-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij��ɫ������Һ�У�Ca2+��NH4+��CO32-��HCOO- | |
| B�� | �ܽ���AlCl3����Һ�У�Na+��K+��SO42-��S2- | |
| C�� | 25��ʱ��ˮ�������c��H+��=1��l0-l3mol/L����Һ�У�K+��Ba2+��NO3-��I- | |
| D�� | ����Ũ�Ⱦ�Ϊ0.1mol/L����Һ�У�Na+��Fe3+��CH3COO-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| װ�� | ʵ����� | �Թ��е�ҩƷ | ���� |
 | ʵ��� | 2mL������Һ���� �ν�ŨNaOH��Һ | �����ݲ����� һ��ʱ�����Һ ��ڣ��Թܱ� �������� |
| ʵ��� | 2mL������Һ�� ����Ũ��ˮ | �����ݲ����� һ��ʱ�����Һ �����Ա仯 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  Al��OH��3 | B�� |  C6H12O6 | C�� |  CH3CH2OH | D�� |  NaHCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 4�� | B�� | 6�� | C�� | 8�� | D�� | 9�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com