
ˮ������֮Դ��Ҳ����Ҫ�Ļ���Ҫ��֮һ��
(1)�۲���ͼ(��)������1 Lˮ�����������ϵ���ˮ����Ҫ�ȽϾ�ȷ����ȡ�����ϵĵ�ˮ����Ӧѡ���������__________��
| A��100 mL�ձ� | B��50 mL�ձ� | C��100 mL��Ͳ | D��50 mL��Ͳ |


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ѧ��ҵ�ǹ��õ�֧����ҵ���������������в��漰��ѧ�仯����
| A�����ʳ��������͵����ϳɰ� | B�����������Ȼ�ԭ��ұ���� |
| C����ҵ�ϳ��ýӴ����������� | D�����ͳ��÷����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ҹ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ��������ͼ��ʾ(ͼ��ijЩת�����輰������δ�г�)��
��д���пհף�
(1)��֪0.5 mol�����0.5 molˮ������t �棬p k Paʱ����ȫ��Ӧ����һ����̼������(�ϳ���)��������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
(2)���������У���ҵ�Ϸ���H2 ��CO2�����ķ����� ��
A�������ͨ������������Һ��������Һ�м�����
B�������ѹ��ȴ��ʹCO2Һ��
C������ð�ˮϴ��
D�������ͨ�뵽ʯ�ҽ���Ȼ��������չ��壬
(3)Ϊ�˱�֤����˳���ϳɣ��ڿ�������ϳ���֮ǰ����Կ������� ��Ŀ����____________________���ںϳɰ���ʵ�����������У�����ȡ�����ɵİ��ӻ�������з����������������ķ��� ��
(4)������������Դ����������߾���Ч�棬����Ҳ�Ƕ���ᡢ��ȫ���ฺ��ı��֣�����ͼ�е��������Ժ���������Դ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧ-ѡ��2��ѧ�뼼������15�֣�
��ͼ���ֽ�����������������������ͼ��
��1���ڢܴ����������������������ܵ��豸������ ���ô�������Ӧ�ķ���ʽΪ ��Ϊ�����������IJ��ʣ��ô�Ӧ���� ������¹��̡����ȹ��̡���Ϊ�ˣ�
��2���ڢߴ����ж��δ�������ԭ���� ��
��3���ݴ�����������Ҫ�ǵ�������������ʱ���徭�����������������Ϊ�� ��
��4��20���ķ������ᣨSO3����������Ϊ20����1�����ˮ �֣�����2λ��Ч���֣��������Ƴ�98���ij�Ʒ���ᣮ
��5���ڢڴ�����1500��ġ�����ȫȼ�ա������Ȼ����������������Ȼ���ڢ۴���700�����ټ���ȼ�գ��Լ�������ȼ�շ�ʽ�Ի���������������ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������ʳ�����������ӣ�ũҵ�Ի�ѧ���ϵ�����Խ��Խ��������������һ�ֻ��ʡ����ʵ������ͺ���ʩ����ũҵ�����������ش����á��ϳɰ���������������ʾ��ͼ���£�
(1)Ŀǰ����ҵ��������ý����������20��50 MPa��450�������õ�������������________�н��кϳɰ�������X�����ȥ��������________��
(2)Ŀǰ����������������CO2Ϊԭ�ϣ�������Ӧ�Ļ�ѧ����ʽ��________��________��
���������ڸ��������������������ط���ˮ�⣬��ˮ��Ļ�ѧ����ʽ��________��
(3)�������з�����Ӧ�Ļ�ѧ����ʽ��________________��
(4)���������еĸ���Ʒ�Ȼ�麟���ʹ�û���������ữ����Ӳ������ᡣ������������Խ��вⶨ������DZ���________�����������������Ч;�����ⶨ������pH�ķ�����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʯ��Ҳ�����ڹ�ҵ���������������������ᡣ��Ȼʯ��Ļ�ѧʽΪCaSO4��2H2O������Ȼʯ����ˮ��2����ѧ��Ӧ�Ϳ��Ƶö�������
(1)д����1����Ӧ�Ļ�ѧ����ʽ__________________________________��
(2)�ڵ�2����ѧ��Ӧ�У�����ԭ�Ͽ���ʹ�õ���(��������)__________��д���йػ�ѧ��Ӧ�ķ���ʽ_______________________________
�ٿ������������������ᡡ�ܽ�̿
(3)�ڵ�2����ѧ��Ӧ�У����ܻ������������̬��Ⱦ����________����(������)����ҪΣ����______________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��12�֣�����ѧ������ѧ�뼼�����ϳɰ���ҵ�����Ṥҵ�����Ṥҵ�ǻ�ѧ��ҵ����Ҫ��ɲ��֡���ش��������⣺
��1����ͼ�ǹ�ҵ�����������ʾ��ͼ��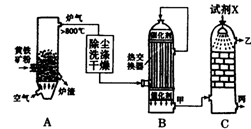
���豸A������Ϊ ��
���Լ�XΪ ��
��B�豸���Ƚ������������� ��
��2����ҵ������ʱ�����������е�ˮ����NO2�Ƿ��ȷ�Ӧ��ΪʹNO2���ñ����գ��ɲ�ȡ��ʩ�� ����ҵ����Na2 CO3��Һ����NO2�Ļ�ѧ����ʽΪ ��
��3����ҵ�ϳɰ��Ļ�ѧ����ʽΪ ��ѡ����Ȼ����Ϊ�ϳɰ�ԭ�������ŵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CO2+H2O=H2CO3 |
| B��H2CO3=CO2��+H2O |
| C��2H2O=O2��+2H2�� |
| D��CaCO3+2HCl=CaCl2+H2O+CO2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����塣�����ܱ������еķ�Ӧ��N2(g)��3H2(g) 2NH3(g)��673 K��30 MPa��n(NH3)��n(H2)��ʱ��仯�Ĺ�ϵ����ͼ��ʾ������������ȷ���� (����)��
2NH3(g)��673 K��30 MPa��n(NH3)��n(H2)��ʱ��仯�Ĺ�ϵ����ͼ��ʾ������������ȷ���� (����)��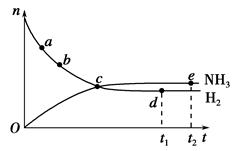
| A����a������Ӧ���ʱȵ�b��С |
| B����c����Ӧ�ﵽƽ�� |
| C����d(t1ʱ��)�͵�e(t2ʱ��)��n(N2)��һ�� |
| D�������������䣬773 K�·�Ӧ��t1ʱ�̣�n(H2)����ͼ��d���ֵ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com