
ij��������X�������й�ϵͼ������A��B�ֱ���X�������ۡ������۽������ӣ���ش�
��1��д��X������ ��Y�Ļ�ѧ ʽ ��
ʽ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
�� X��ϡ���ᷴӦ����A��ij����������ӷ���ʽ
�� +2�۵�A��Y������Ӧ����+3�۵�B�����ӷ���ʽ
�� X��ĩ������ͭ��Һ�����û���Ӧ�����ӷ���ʽ

�� X�������������X2O3�������۷�Ӧ�Ļ�ѧ����ʽ
��3��A��Һ��NaOH��Һ�ڿ����з�Ӧ������  ��
��
��д���йصĻ�ѧ����ʽ ��
 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| ||
| ||
| 168Q |
| m |
| 168Q |
| m |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
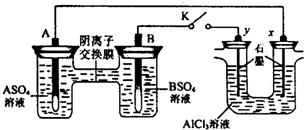
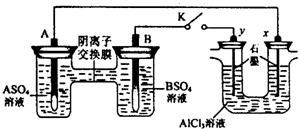
| A����Һ��c��A2+��Ũ�ȼ�С | B��B�ĵ缫��Ӧ��B-2e-?B2+ | C��y�缫����H2������������ԭ��Ӧ | D����Ӧ���ڣ�x�缫��Χ���ְ�ɫ��״���������ó����ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
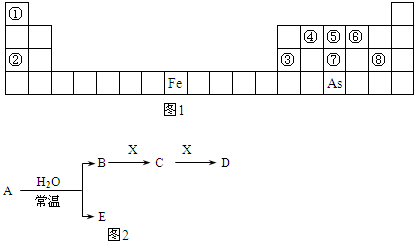
ij��������X�������й�ϵͼ������A��B�ֱ���X�������ۡ������۽������ӣ���ش�

��1��д��X������ ��Y�Ļ�ѧʽ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
�� X��ϡ���ᷴӦ����A��ij����������ӷ���ʽ
�� +2�۵�A��Y������Ӧ����+3�۵�B�����ӷ���ʽ
�� X��ĩ������ͭ��Һ�����û���Ӧ�����ӷ���ʽ
�� X�������������X2O3�������۷�Ӧ�Ļ�ѧ����ʽ
��3��A��Һ��NaOH��Һ�ڿ����з�Ӧ������ ��
��д���йصĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com