




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| | �������↑ʼ����ʱ��pH | �������������ȫʱ��pH | ��ѡ���Լ� |
| Fe3+ | 1.9 | 3.7 | A.��ˮ�� B.H2O2�� C.NaOH�� D.��ˮ�� E.CuO�� F.Cu2(OH)2CO3 |
| Fe2+ | 7.5 | 11 | |
| Cu2+ | 6.0 | 10 |
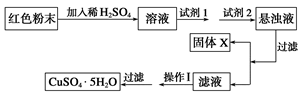
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
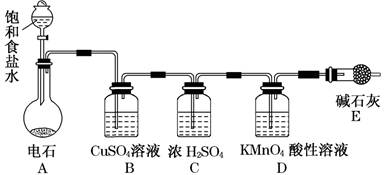
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ƽ����25.20g�Ȼ��ƹ��� |
| B����250ml����ƿ����150mLһ��Ũ�ȵ�������Һ |
| C����һ��ǿ�����侭����е����Ȼ�����Һ�й�����ͨ·��˵�������������������� |
| D����������˿�������е�ȼ������ȡ�Ȼ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
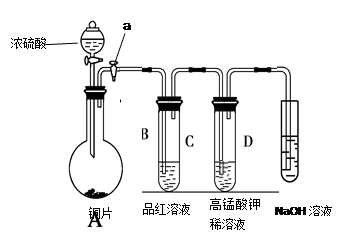
| ��� | ʵ����� | Ԥ������ͽ��� |
| ����� | | �а�ɫ�������ɣ�֤������Һ�к�SO42���� |
| ����� | | |
| ����� | | |
| ���� | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
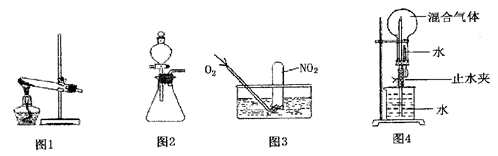
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
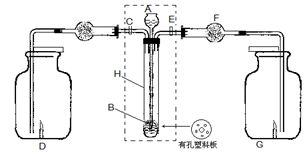
 ����Ӧ����Ʋ��������ͼ��ʾ
����Ӧ����Ʋ��������ͼ��ʾ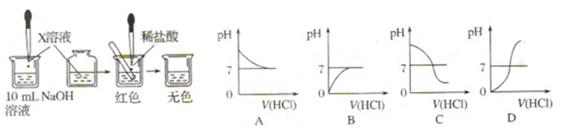
 �Թ��У��ý�ͷ�ι���������ϡ���ᣬ��������Һ��ͬʱ�ⶨ��Һ��pH��ֱ�����������
�Թ��У��ý�ͷ�ι���������ϡ���ᣬ��������Һ��ͬʱ�ⶨ��Һ��pH��ֱ�����������| ��������� ���( 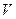 )/mL )/mL | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 |
| ��Һ�¶����� | 5.2 | 9.6 | 12.0 | 16.0 | 18.2 | 16.7 | 15.7 | 14.7 | 13.7 |
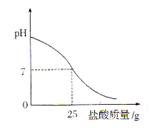
 ������������������֮��ı仯��ϵͼ�����������ϻ����ܱ�ʾ����֮��ǡ����ȫ��Ӧ�ĵ㣬������ĸP��ʾ��
������������������֮��ı仯��ϵͼ�����������ϻ����ܱ�ʾ����֮��ǡ����ȫ��Ӧ�ĵ㣬������ĸP��ʾ��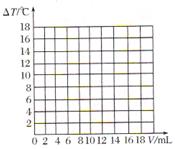
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com