���㣺�����ʱ�Ķ����жϼ��й�ph�ļ���,Ӱ������ˮ��̶ȵ���Ҫ����
ר�⣺����ƽ������Һ��pHר��,�����ˮ��ר��
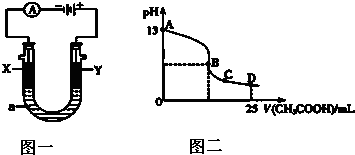
��������1����ͼӦ��ʵ���������Ӻ����������ӵķ�Ӧ����pH�������Ba��OH��
2��NaOH��NH
3?H
2O������Һ������������Ũ����ȣ���һˮ�ϰ����ܵ�������������ӣ�������ʹ�õ�����ࣻ��ͼӦ��ʵ���������Ӻ����������ӵķ�Ӧ������������ʵ���Ũ�ȵ�Ba��OH��
2��NaOH��NH
3?H
2O������Һ�ֱ�͵�Ũ�ȵ������ϣ��һ����Һ�����ԣ��ܵ�������������ӵ����ʵ���Խ�࣬��Ҫ�������Խ���Ȼ�淋���Һ�����ԣ�Ҫʹ�����Һ�����ԣ����������Ӧ������Щ��
��2����a mol?L
-1�İ�ˮ��b mol?L
-1������������ϣ���Ӧ����Һ�����ԣ���Һ��c��OH
-��=1��10
-7mol/L�����ݰ�ˮ�ĵ���ƽ�ⳣ������ʽ�����K
b��
��3����ˮ��NH
4Cl�����ʵ���������Ƴɵ�ϡ��Һ��c��Cl
-����c��NH
4+�������ݵ���غ��֪����Һ��c��OH
-����c��H
+������Һ��ʾ���ԣ�Ȼ������ε�ˮ�⡢������ʵ��롢����غ㡢�����غ�����жϣ�
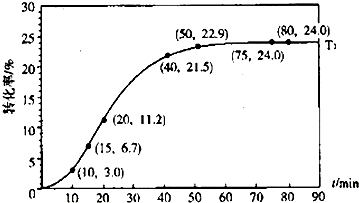
��1����ͼ��������֪����75minʱ�ﵽƽ�⣬����ij����ת����Ϊ24%�������ƽ��ʱ��Ӧ���������ʵ����ʵ������������ʽ���㣻
��2����ͼ����֪��15minʱ������ij����ת����Ϊ6.7%��20minʱ������ij����ת����Ϊ11.2%������ʱ��ʣ��ļ���ij�������ʵ���֮���Ϊ15��20min��Χ�ڼ���ij���ļ�������������Ŀͼ����֪��Ӧ����Ϊ��λʱ�������ʵ����ı仯��15��20min��Χ�ڼ���ij����ƽ������Ϊ
���ݴ���⣻
��3��������д����ã��ӿ췴Ӧ����ʼ������Ũ��С����Ч�������ԣ����ŷ�Ӧ����������Ũ������Ч�����ԣ��������ӵ�һ���̶Ⱥ�Ũ�ȶԷ�Ӧ���ʵ�Ӱ����������أ��ر����淴Ӧ���ʵ������ﵽƽ��ʱ����Ӧ���ʼ������䣮
���
�⣺��1����ͼӦ��ʵ���������Ӻ����������ӵķ�Ӧ����pH�������Ba��OH��
2��NaOH��NH
3?H
2O������Һ������������Ũ����ȣ���һˮ�ϰ����ܵ�������������ӣ�������ʹ�õ�����࣬��������������������Һʹ�õ�����������ȣ���V
1 =V
2��V
3��
��ͼӦ��ʵ���������Ӻ����������ӵķ�Ӧ������������ʵ���Ũ�ȵ�Ba��OH��
2��NaOH��NH
3?H
2O������Һ�ֱ�͵�Ũ�ȵ������ϣ��һ����Һ�����ԣ��ܵ�������������ӵ����ʵ���Խ�࣬��Ҫ�������Խ�����ʵ�������������Һ��������������������ʵ���������������������ˮ��Ҫ�������С���������ƣ�������Ҫ���������С˳����V
1 ��V
2��V
3��
�ʴ�Ϊ��V
1 =V
2��V
3��V
1 ��V
2��V
3��
��2����a mol?L
-1�İ�ˮ��b mol?L
-1������������ϣ���Ӧ����Һ�����ԣ���Һ��c��OH
-��=1��10
-7mol/L��
��Һ��c��NH
4+��=c��Cl
-��=
mol/L����Ϻ�Ӧǰc��NH
3?H
2O��=
mol/L����Ӧ��c��NH
3?H
2O��=��
-
��mol/L��K
b=
=
=10
-7��
�ʴ�Ϊ��10
-7��
��3����ˮ��NH
4Cl�����ʵ���������Ƴɵ�ϡ��Һ��c��Cl
-����c��NH
4+�������ݵ���غ��֪��c��OH
-����c��H
+����
A����ˮ�ĵ������ô���NH
4Cl��ˮ�����ã�������Һ��ʾ���ԣ�˵����ˮ�ĵ���̶ȴ���笠����ӵ�ˮ��̶ȣ���A��ȷ��
B����ˮ�ĵ�������С��NH
4Cl��ˮ�����ã�����A��֪����ˮ�ĵ���̶ȴ����Ȼ�淋�ˮ��̶ȣ���B����
C����ˮ�Ĵ���������NH
4Cl��ˮ�⣺笠����Ӵ���ˮ��ƽ�⣬��ˮ�����笠����Ӻ����������ӣ���Һ��笠�����Ũ�������������Ȼ����笠����ӵ�ˮ��̶ȣ���C��ȷ��
D��NH
4Cl�Ĵ��������˰�ˮ�ĵ��룺�Ȼ�淋����笠����ӣ�ʹ��Һ��笠�����Ũ������ˮ�ĵ���̶ȼ�С���Ȼ�������˰�ˮ�ĵ��룬��D��ȷ��
E��c��H
+����c��OH
-��������c��Cl
-����c��NH
4+�������ݵ���غ��֪��c��OH
-����c��H
+������E����
F��c��NH
3?H
2O����c��NH
4+�������ڰ�ˮ�ĵ���̶ȴ���笠����ӵ�ˮ��̶ȣ���c��NH
3?H
2O����c��NH
4+������F����
G��c��NH
3?H
2O��+c��NH
4+��=2c��Cl
-�������������غ�ɵã�c��NH
3?H
2O��+c��NH
4+��=2c��Cl
-������G��ȷ��
H��c��NH
3?H
2O��+c��OH
-��=c��Cl
-��+c��H
+�������ݵ���غ�ɵã���c��NH
4+��+c��H
+��=c��Cl
-��+c��OH
-�������������غ㣺��c��NH
3?H
2O��+c��NH
4+��=2c��Cl
-��������-�ٿɵã�c��NH
3?H
2O��+c��OH
-��=c��Cl
-��+c��H
+������H��ȷ��
�ʴ�Ϊ��BEF��
��1��ij�ּ�����ˮ�ⷴӦ����ʽΪ��HCOOR��l��+H
2O��l��?HCOOH��l��+ROH ��l������ͼ��������֪����75minʱ�ﵽƽ�⣬����ij����ת����Ϊ24%�����Լ���ij��ת�������ʵ���Ϊ1.00��24%=0.24mol����Ϸ���ʽ�ɼ����ƽ��ʱ������ij�����ʵ���=0.76mol��ˮ�����ʵ���1.75mol����������ʵ���=0.25mol��ij�������ʵ���=0.76mol��
�ʴ�Ϊ��0.76��
��2��15minʱ������ij����ת����Ϊ6.7%������15minʱ������ij����Ũ��Ϊ����1-1.00��6.7%��mol/L=0.933mol/L��20minʱ�����������ת����Ϊ11.2%����20minʱ�����������Ũ��Ϊ��1-1.00��11.2%��mol/L=0.888mol/L������15��20min��������ļ�����Ϊ0.933mol/L-0.888mol/L=0.045mol/L������������ƽ������=
=0.009mol?L
-1?min
-1��
�ʴ�Ϊ��0.009��
��3����������ݲ��ѿ�����ƽ�����ʵı仯��ת���ʵ������������ټ�С���ֲ��䣮��Ϊ��Ӧ��ʼ���������Ũ�ȴ����Է�Ӧ���ʽϴ����ŷ�Ӧ���м��������Ũ�ȼ�С����Ӧ���ʼ�С�����ﵽƽ��ʱ����Ӧ���ʼ������䣻�ٷ�Ӧ���ڣ���Ȼ������������ϴ���������С����Ч�������ԣ���Ӧ���ʽ������ڷ�Ӧ���ڣ������������࣬��Ч����������Ӧ�����������۷�Ӧ���ڣ����������ӵ�һ���̶Ⱥ�Ũ�ȶԷ�Ӧ���ʵ�Ӱ����������أ��ر����淴Ӧ���ʵ�����ʹ�ܷ�Ӧ������С��ֱ��Ϊ�㣬
�ʴ�Ϊ��������
���������⿼��������ϵĶ����жϼ���Һ�������pH�ļ��㡢�ε�ˮ��ԭ����������ʵĵ��뼰��Ӱ�����أ���Ŀ�ѶȽϴ����������ϴ�ֿ�����ѧ������ѧ֪ʶ�����������