
��10�֣����亽�������õ����⻯���£�N2H4����ȼ�ϡ��Իش������й����⣺
��1��N2H4ȼ��ʱ��NO2�����������������Ӧ���ɵ�����ˮ������
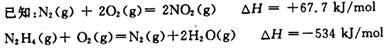
��N2H4��NO2��Ӧ���ɵ�����ˮ�������Ȼ�ѧ����ʽΪ ��
��2�������¡�������KOH��Һ��ɼ���ȼ�ϵ�أ���Ԫ�ر�������ֻ���ɵ�������д���õ�ع���ʱ�����ĵ缫��Ӧ ����ع���ʱ�� ��pH���ߡ�
��3����ͳ�Ʊ��µķ���������NaClO����NH3���Ƶ��µ�ϡ��Һ���÷�Ӧ�Ļ�ѧ����ʽ ��
��4�������£�N2H6Cl2����һ����Ҫ�Ļ���ԭ�ϣ��������ӻ����������ˮ����Һ�����ԣ�ˮ��ԭ����NH4Cl���ơ�д�������µ�һ��ˮ�ⷴӦ�����ӷ���ʽ ��
 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ���¿α�2007��߿���ѧģ������(ɽ����) ���ͣ�022
| |||||||||||||||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)д��N2�ĵ���ʽ_________________________________��
(2)Һ̬NH3����H2O��Ҳ���������Ҳ�����������ͬ���������ӣ���Һ̬NH3���뷽��ʽΪ_________________________________��
(3)25��ʱ��0.1 mol.L-1NH4NO3��Һ��ˮ�ĵ���̶�___________(����ڡ������ڡ���С�ڡ�)0.1 mol��L-1NaOH��Һ��ˮ�ĵ���̶ȡ�����0.1 mOl��L-1NaOH��0.2 m01.L-1 NH4NO3����Һ�������ϣ�������Һ������Ũ���ɴ�С��˳��Ϊ______________________��
(4)�ڽṹ��N2H4��NH3�Ĺ�ϵ����H2O2��H2O�Ĺ�ϵ��N2H4�ܷ������з�Ӧ��
N2H4+H3O+====![]() + H2O
+ H2O
N2H4+ H2O![]()
![]() + OH-
+ OH-
![]() +H2O
+H2O![]()
![]() +OH-
+OH-
![]() +H2O
+H2O![]() N2H4+H3O+
N2H4+H3O+
�ݴ˿ɵó��Ľ�����__________________��
A.��ˮ�������� B.����ˮ�е����H+����
C.���Ƕ�Ԫ���� D.���Ƕ�Ԫ����
(5)������з�Ӧ����ʽ����ƽ��
______NH3+______NaClO====NaCl+______N2H4+______ ______
(6)������ʱ��(N2H4)Ϊȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ����֪4gN2H4(g)��������Ӧ�зų�71kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ)___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���亽�������õ����⻯���£�N2H4����ȼ�ϡ��Իش������й����⣺
��1��д��N2�ĵ���ʽ
��2��Һ̬NH3����H2O��Ҳ���������Ҳ�����������ͬ���������ӣ���Һ̬NH3���뷽��ʽΪ_________________________________��
��3��25��ʱ��0.1 mol��L��1 NH4NO3��Һ��ˮ�ĵ���̶�________________ ������ڡ��������ڡ���С�ڡ���0.1 mol��L��l NaOH��Һ��ˮ�ĵ���̶ȡ�����0.1mol��L��1NaOH��0.2mol��L��1 NH4NO3����Һ�������ϣ�������Һ������Ũ���ɴ�С��˳��Ϊ________________��
��4���ڽṹ��N2H4��NH3�Ĺ�ϵ����H2O2��H2O�Ĺ�ϵ��N2H4�ܷ������з�Ӧ��
N2H4+H3O+==N2H5��+H2O N2H4+H2O![]() N2H5��+OH��
N2H5��+OH��
N2H5��+H2O![]() N2H62��+OH�� N2H5��+ H2O
N2H62��+OH�� N2H5��+ H2O![]() N2H4+H3O+
N2H4+H3O+
�ݴ˿ɵó��Ľ�����______________________��
A����ˮ�������� B������ˮ�е����H+����
C�����Ƕ�Ԫ���� D�����Ƕ�Ԫ����
��5��������ʱ�£�N2H4��Ϊȼ�ϣ�˫��ˮ�������������߷�Ӧ���ɵ�������̬ˮ����֪3.2gN2H4(l)��������Ӧ�зų�64.22kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ
________ ��
����¿�����Ϊ����ƽ��������ݸ÷�Ӧ������Ϊ�Ƿ����ͨ���ı䷴Ӧ��������N2��ˮ����ȡN2H4�����ܣ�ָ�����ܵķ�Ӧ�����������ܣ���ָ��ԭ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���亽�������õ����⻯���£�N2H4����ȼ�ϡ��Իش������й����⣺
��1��д��N2�ĵ���ʽ______________��
��2��Һ̬NH3����H2O��Ҳ���������Ҳ�����������ͬ���������ӣ���Һ̬NH3���뷽��ʽΪ______________________________��
��3��25��ʱ��0��1 mol?L-1 NH4NO3��Һ��ˮ�ĵ���̶�_______������ڡ��������ڡ���С�ڡ���0��1 mol?L-l NaOH��Һ��ˮ�ĵ���̶ȡ�����0��1mol?L-1NaOH��0��2mol?L-1 NH4NO3����Һ�������ϣ�������Һ������Ũ���ɴ�С��˳��Ϊ_________��
��4���ڽṹ��N2H4��NH3�Ĺ�ϵ����H2O2��H2O�Ĺ�ϵ��N2H4�ܷ������з�Ӧ��
N2H4+H3O+==N2H![]() +H2O�� ������������N2H4+H2O
+H2O�� ������������N2H4+H2O![]() N2H
N2H![]() +OH-
+OH-
N2H![]() +H2O
+H2O![]() N2H
N2H![]() +OH-�� ����������N2H
+OH-�� ����������N2H![]() + H2O
+ H2O![]() N2H4+H3O+
N2H4+H3O+
�ݴ˿ɵó��Ľ�����______________________��
A����ˮ�������ԡ� ���������� B������ˮ�е����H+����
C�����Ƕ�Ԫ��������������� D�����Ƕ�Ԫ����
��5��������з�Ӧ����ʽ����ƽ��
______NH3+______NaClO==_________NaCl+_______N2H4+_____ _____
��6��������ʱ�ݣ�N2+H4��Ϊȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ����֪4gN2H4��g����������Ӧ�зų�71kJ��������д���÷�Ӧ��ת��ѧ����ʽ___________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com