
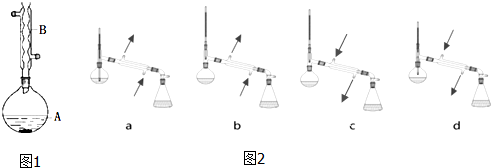
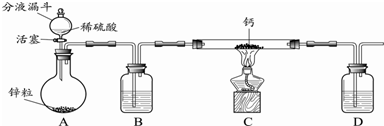
���� ��װ��A�м��뷴Ӧ������2��3Ƭ���Ƭ����ʼ��������A��������������������50���ӣ���ӦҺ�������º����Һ©���У���������ˮϴ��������ʹ��ᣬ����ˮϴ��̼��������Һ���ֳ��IJ������������ˮ����þ�����������������Ƭ�̣����˳�ȥ����þ���壬�����������ռ�140��143����֣����������������������˷�Ӧ��ת���ʵ���߷�����ͬʱ���в��ʵļ�������������
��1��������������B�Ĺ����жϸ����������ƣ�
��2�������ǻ��������⣬�������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ��Ũ����Ϊ��������ˮ����
��3��������Ӧ�ı���Ϊ�����ǻ��������⣬�������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ��
��4�������������������ܶȼ���ȷ�ķ�Һ�����������н��
��5��������ˮ����þ�ܹ�����������������������ˮ�֣��������ã�
��6���ȸ����¶ȼ�����������е������ų�ad���ٸ�����������������ʹ��Ʒ����������ȫ���ռ�����ƿ�У��ó���ȷ���ۣ�
��7�����жϹ�����������ݷ�Ӧ����ʽ���������������������������������Ȼ���������������IJ���
��� �⣺��1����װ��������B�Ĺ����֪������B������Ϊ���������ܣ�
�ʴ�Ϊ�����������ܣ�
��2�������ǻ��������⣬�������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ��Ũ����Ϊ��������ˮ����
��3��������Ӧ�ı���Ϊ�����ǻ��������⣬�������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ���÷�ӦΪ���淴ӦΪ��CH3COOH+C2H5OH$?_{��}^{Ũ����}$CH3COOC2H5+H2O��
�ʴ�Ϊ��CH3COOH+C2H5OH$?_{��}^{Ũ����}$CH3COOC2H5+H2O��
��4�����������ܶȱ�ˮС�����������ܣ����ˮ���²㣬�����ϲ㣻��Һʱ��Ҫ�Ƚ�ˮ��ӷ�Һ©�����¿ڷų�����������Һ�����ʱ�رշ�Һ©���Ļ������ٽ��������������Ͽڷų���������ȷ��ΪD��
�ʴ�Ϊ��D��
��5��ʵ���м���������ˮ����þ��Ŀ������������������ˮ�֣����������������и��
�ʴ�Ϊ�����
��6������������У��¶ȼƵ�ˮ����Ҫ����������ƿ��֧�ܿڴ�������ad����c��ʹ�õ�����������������ʹ��Ʒ����������ȫ���ռ�����ƿ�У����������װ�ð�װ��ȷ����b��
�ʴ�Ϊ��b��
��7��4.6g�Ҵ������ʵ���Ϊ0.1mol��9g��������ʵ�����0.15mol��0.1mol�Ҵ���ȫ��Ӧ����0.1mol�����ᣬ������ʣ�࣬������Ҫ�����Ҵ��������������ɵ������������������ݷ�ӦCH3COOH+CH3CH2OH$\frac{\underline{\;Ũ����\;}}{��}$CH3COOCH2CH3+H2O��֪�������������������������ʵ���Ϊ0.1mol����8.8g��ʵ��������5.28g�������������IJ���Ϊ��$\frac{5.25g}{8.8g}��100%$��60%
�ʴ�Ϊ��d
���� ����Ϊһ���߿������⣬�����˳��������Ĺ����밲װ�������ķ��롢�ᴿ�����ʵ���ȡ��ҩƷ��ѡ��ʹ�á����ʲ��ʵļ����֪ʶ����Ŀ�ѶȽϴ������漰�������ϴ�֪ʶ��϶࣬���������ѧ���ķ������������������Ӧ����ѧ֪ʶ��������


 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�갲��ʦ���и߶���10���¿���ѧ���������棩 ���ͣ�ѡ����
��һ �������£�����A2��g��+3B2��g��
�������£�����A2��g��+3B2��g�� 2AB3��g����Ӧ��˵����������ʾ�Ļ�ѧ��Ӧ����������
2AB3��g����Ӧ��˵����������ʾ�Ļ�ѧ��Ӧ���������� ��
��
A��v��A2��=0.8 mol��L-1��s-1 B��v��A2��=40 mol��L-1��min-1
C��v��AB3��=1.0 mol��L-1��s-1 D��v��B2��=1.2 mol��L-1��s-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g•cm-3�� |
| �Ҵ� | -117.3 | 78.5 | 0.79 |
| ���� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.90 |
| Ũ���� | - | 338.0 | 1.84 |
�鿴�𰸺ͽ���>>
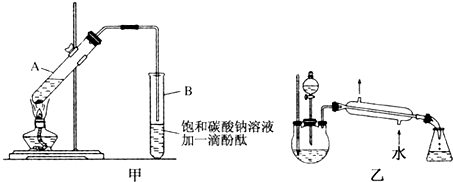
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A��ʢ��Ũ���ᣬB��ʢ����ˮ�Ҵ��ͱ����ᣮ
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A��ʢ��Ũ���ᣬB��ʢ����ˮ�Ҵ��ͱ����ᣮ| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е㣨�棩 | 34.7 | 78.5 | 118 | 77.1 |
 CH3CO18OCH2CH3+H2O��
CH3CO18OCH2CH3+H2O���鿴�𰸺ͽ���>>
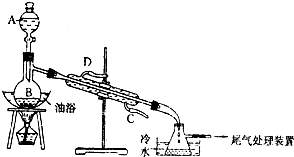
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ijѧ����ʵ����������ͼ��ʵ��װ���Ʊ������������о��䷴Ӧ������
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ijѧ����ʵ����������ͼ��ʵ��װ���Ʊ������������о��䷴Ӧ������| ���� | �е�/��C | �ܶ�/g?cm-3 |
| �Ҵ� | 78.0 | 0.79 |
| ���� | 117.9 | 1.05 |
| �������� | 77.5 | 0.90 |
| ���촼 | 131 | 0.8123 |
| ���������� | 142 | 0.8670 |
| ʵ���� | �Թܢ��е��Լ� | ����л���ĺ��/cm |
| A | 2mL�Ҵ���2mL���ᡢ1mL 18mol/LŨ���� | 5.0 |
| B | 3mL�Ҵ���2mL���� | 0.1 |
| C | 3mL�Ҵ���2mL���ᡢ6mL 3mol/L���� | 1.2 |
| D | 3mL�Ҵ���2mL���ᡢ���� | 1.2 |
�鿴�𰸺ͽ���>>
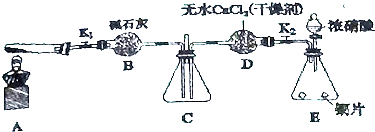
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
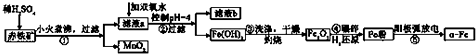
| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
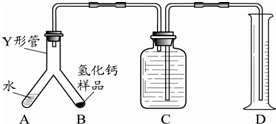
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com