
���п�ͼ��ʾ��ת����ϵ�У�A��C��Ϊ�����Ľ������ʣ�A��C�����H��Ũ��Һ�о��ᷢ���ۻ���EΪ����ǽ������ʡ�BΪ��ɫ���������XΪ������ɫҺ�塣L��ɫΪ��ɫ������ʹ��̪��죨��Ӧ���������ɵ�ˮ��������������ȥ����

��ش��������⣺
��1����̼����0.03 %��2 %֮���C�ĺϽ���Ŀǰ������ʹ�������ĺϽ����ֺϽ��� ��
A�����Ͻ� B����ͭ C��þ�Ͻ� D������
��2��F�Ļ�ѧʽΪ ��
ȡ�����μ�KSCN��Һ����Һ��Ѫ��ɫ�����ɫ��Һ��������ΪFe3+
��3��I��������Ӧ�����ӷ���ʽΪ ��
��4��A��B����C��D�ķ�Ӧ�� ����ų��������ա������������ķ�Ӧ��
��5��D��L��Ӧ�����ӷ���ʽΪ ��
Al2O3+2NaOH=2NaAlO2+H2O

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ᡢ��ȩ������������CH3COOCH2CH3������ϩ��ɵĻ�����У��������������������Ϊ15.3�����������������Ϊ
A��84.7 �� B��72.6 �� C��12.1 �� D��6.05 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ȤС�����������ʵ��װ�á�ʵ��ʱ���ȶϿ�K2���պ�K1�������������ݲ�����һ��ʱ��Ͽ�K1���պ�K2���� �ֵ�����ָ��ƫת�������й�������ȷ����
�ֵ�����ָ��ƫת�������й�������ȷ����

A���Ͽ�K2���պ�K1ʱ���ܷ�Ӧ�����ӷ���ʽΪ��2H++2Cl��  Cl2��+H2��
Cl2��+H2��
B���Ͽ�K2���պ�K1ʱ��ʯī�缫������Һ���
C���Ͽ�K1���պ�K2ʱ��ʯī�缫������
D���Ͽ�K1���պ�K2ʱ��ͭ�缫�ϵĵ缫��ӦΪ��Cl2+2e��=== 2Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ж��й�ʵ����ʵ�Ľ�����ȷ����
A����ij��Һ�еμ���ˮ���ټ���KSCN��Һ����Һ�ʺ�ɫ��˵��ԭ��Һ�к���Fe2+
B��Ũ�����Ũ���᳤�ڱ�¶�ڿ�����Ũ�Ⱦ����ͣ�ԭ������ͬ
C����ij��Һ�м����Ȼ�����Һ�����ɰ�ɫ�������ټ���ϡ���ᣬ�������ܽ⣬��ԭ��Һһ������SO42
D�������£�Ũ��������������������ˣ�˵���������Ũ�����Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A�������ڷ�Ӧ��ֻ������ԭ�����ǽ����ڷ�Ӧ��ֻ����������
B���������ڷ�Ӧ��ʧȥ���ӣ���ԭ���ڷ�Ӧ�еõ�����
C�����������������ԣ���ԭ�����л�ԭ��
D��������ֻ�������ԣ�������ֻ�л�ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���������ȡҺ ���Լ�����ȡ�����Լ������ܽ⣬�ܹ��ˣ���������һ��Ҫ�õ����������ǣ� ��
���Լ�����ȡ�����Լ������ܽ⣬�ܹ��ˣ���������һ��Ҫ�õ����������ǣ� ��
A. �٢ڢ� B.�ڢۢ� C. �٢ڢ� D. �ۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪1.505��1023��X������ӵ�����Ϊ8 g����X�����Ħ��������(����)
A��16 g B��32 g
C��64 g/mol D��32 g/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼΪ����250 mL 0.2 mol/L Na2CO3��Һ��ʾ��ͼ��
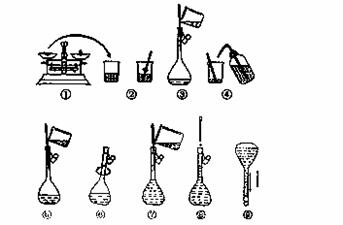
�ش��������⣺
(1)���гƵ�Na2CO3________g��ѡȡ����ƿ���______________
(2)����ƿʹ��ǰ����©ˮ�ķ����� ____________________��
(3)�����������������������ҺŨ���к�Ӱ�죿(�ƫ�ߡ���ƫ�͡�����Ӱ�족)
A��ijͬѧ�ڵڢಽ�۲�Һ��ʱ����________��
B��û�н��в������� �ܺ͢�________��
�ܺ͢�________��
C���ڵڢݲ�����������Һ����������ƿ�� ________��
D δ����ȴ���Ƚ���Һע������ƿ�ж��� ________��
Eҡ�Ⱥ���Һ����ڿ̶����ټ�ˮ _______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з��ӽṹ�У�����ԭ�Ӳ��ܶ����������Ϊ8�����ȶ��ṹ����
A��CO2 B��H2O C��N2 B��CCl4
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com