
| ������/kJ•mol-1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
| ���ۼ� | C-C | C-N | C-S |
| ����/kJ•mol-1 | 347 | 305 | 259 |

| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ•mol-1 | 786 | 715 | 3401 |
���� ��1������ͬ����Ԫ�صĵ������ж�Ԫ�ص����࣬A��I4������ͻȻ����˵���������3�����ӣ�B��I3������ͻȻ����˵���������2�����ӣ�
��2�����������Ĺ��������е������뵰���ʷ�������Ҫ��ѧ���������������ȽϷ�������İ�����Ϊ�ʰ��
��3�����Ӿ����о�����Խ���γɵ����Ӿ���Խ�ȶ����۵�Խ��Ӳ��Խ�����������ӵİ뾶������йأ����Խ�ࡢ���Ӱ뾶ԽС��������Խ���ݽṹ���������ж�һ��Mg2+��Χ�������ڽ��ҵȾ����Mg2+������
��4�����������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�������ӵ������������ж�δ�ɶԵ��ӣ�
��5����������д��ڵĻ�ѧ���У��ǽ���Ԫ��֮��Ĺ��ۼ�����Ԫ���뵪Ԫ��֮�����λ������ԭ�Ӻ���ԭ��֮��������
��� �⣺��1��A��I4������ͻȻ����˵���������3�����ӣ���AΪ��������Ԫ�أ���AӦΪAl��B��I3������ͻȻ����˵���������2�����ӣ���BΪ��������Ԫ�أ���BӦΪMg��Al�������ϼ�Ϊ+3�ۣ�Mg�ĺ�����12�����ӣ��������Ų���������Ų�ʽΪ��1s22s22p63s2��
�ʴ�Ϊ��+3��1s22s22p63s2��
��2������Ϊ300nm�������Ĺ��������е�����ԼΪ399kJ/mol���ȵ����ʷ�����C-C��C-N��C-S�ļ��ܶ������Բ���Ϊ300nm�������Ĺ������ƻ������ʷ����еĻ�ѧ�����Ӷ��ƻ������ʷ��ӣ���İ�����Ϊ�ʰ��ᣬ�ʰ������Ȼ���̼ԭ��Ϊ sp2 �ӻ�����һ��̼ԭ��Ϊ sp3 �ӻ���
�ʴ�Ϊ���������е������ȵ����ʷ�������Ҫ�Ļ�ѧ��C-C��C-N��C-S�ļ��ܶ�����������������ʹ��Щ��ѧ�����ѣ��Ӷ��ƻ������ʷ��ӣ�sp2��sp3��
��3�����Ӿ����о�����Խ���γɵ����Ӿ���Խ�ȶ����۵�Խ��Ӳ��Խ�����������ӵİ뾶������йأ����Խ�ࡢ���Ӱ뾶ԽС��������Խ��TiN�����������������Ϊ3��������������������ɣ�MgO��CaO�����������ͬ����þ���Ӱ뾶С�ڸ����Ӱ뾶���Ȼ��������������������Ϊ1���Ҽ����Ӱ뾶�������Ӱ뾶�������Ӱ뾶���������Ӱ뾶������KCl��MgO��CaO��TiN4�����Ӿ����۵�Ӹߵ��͵�˳����TiN��MgO��CaO��KCl��
MgO�ľ���ṹ��NaCl�ľ���ṹ���ƣ�����һ��Mg2+��Χ�������ڽ��ҵȾ����Mg2+����Ϊ12��
�ʴ�Ϊ��TiN��MgO��CaO��KCl��12��
��4��V2O5��V����������ȫ��ʧȥ��ɼ���CrO2��Crʧȥ4�����ӣ����ӵ���������Ϊ2��Ϊ�ɶԣ����Ӻ�δ�ɶԵ���Խ�࣬�����Խ�����ʺ���¼�����ŷ�ԭ�ϵ���CrO2��
�ʴ�Ϊ��CrO2��
��5����������д��ڵĻ�ѧ���У��ǽ���Ԫ��֮��Ĺ��ۼ�����Ԫ���뵪Ԫ��֮�����λ������ԭ�Ӻ���ԭ��֮����������ѡAC��
�ʴ�Ϊ��AC��
���� ���⿼���˻��ϼۺ͵縺�Ե��жϡ���ѧ������������Ų�ʽ����д��֪ʶ�㣬�ѶȽϴ�ע�⣨3������Ľṹ��ע�⾧���������Ӿ����۵�Ĺ�ϵ����Ŀ�Ѷ��еȣ�


 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
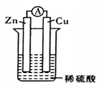
| A�� | ��Һ�е��������������ƶ������������ƶ� | |
| B�� | ����һ��ʱ�ʹ�����'���Һ��pHֵ��С | |
| C�� | пƬ��������ͭƬ�������ݲ��� | |
| D�� | ���������Ǵ�пƬ����ͭƬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��3���ڢ�B�� | B�� | ��4���ڢ�B�� | C�� | ��4���ڢ�B�� | D�� | ��4���ڢ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
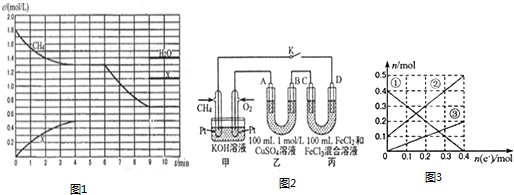
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��ѧ�� | H-H | C-O | C��O | H-O | C-H |
| ����/kJ��mol-1 | a | b | x | c | d |
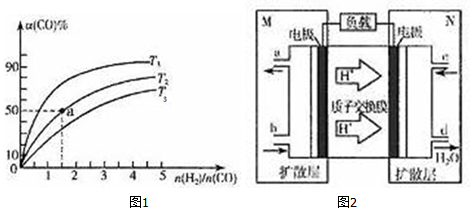
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
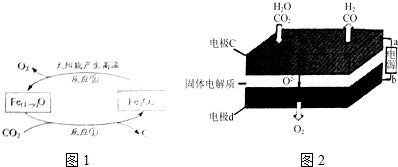
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
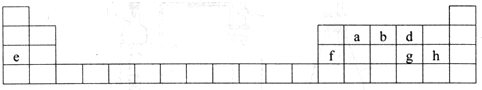
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| t�� | 400 | 500 | 800 | 1000 |
| K | 2.6 | 1.6 | 1.0 | 0.40 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
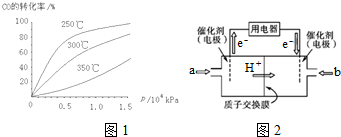
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com