

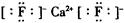 (ÿ��2�֣���8��)
(ÿ��2�֣���8��)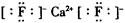 ��
��

 ȫ��������ϵ�д�
ȫ��������ϵ�д� һ��һ����ʱ���ϵ�д�
һ��һ����ʱ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ���: I1=738kJ/mol I2 =" 1451" kJ/mol I3 = 7733kJ/mol I4 = 10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵڰ��� |

 ���е�
���е� ����
���� ����������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж�GE3�ľ�������Ϊ________________��
����������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж�GE3�ľ�������Ϊ________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| H | | | | | | | He |
| 1.3120 | | | | | | | 0.3723 |
| Li | Be | B | C | N | O | F | Ne |
| 0.5203 | 0.8995 | 0.8001 | 1.0864 | 1.4023 | 1.3140 | 1.6810 | 2.0807 |
| Na | Mg | Al | Si | P | S | Cl | Ar |
| 0.4958 | 0.7377 | 0.5776 | 0.7865 | 1.0118 | 0.9996 | 1.2511 | 1.5205 |
| K | Ca | Ga | Ge | As | Se | Br | Kr |
| 0.4189 | 0.5898 | 0.5788 | | 0.9440 | 0.9409 | 1.1399 | 1.3507 |
| Rb | Sr | In | Sn | Sb | Te | I | Xe |
| 0.4030 | 0.5495 | 0.5583 | 0.7086 | 0.8316 | 0.8693 | 1.0084 | 1.1704 |
| Cs | Ba | Tl | Pb | Bi | Po | At | |
 ��ʾ����λ�ã��ڴ���������⡢������ȴ�������ӦSi����
��ʾ����λ�ã��ڴ���������⡢������ȴ�������ӦSi���� ��ʾ����λ�á�
��ʾ����λ�á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����е�ԭ�Ӻ��������������������� |
| B�������ӣ�H+��ʵ������һ����¶������ |
| C����������Ų���ͬ�������仯ѧ����Ҳ��ͬ |
| D���ǽ���Ԫ��ԭ������������������4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Ӽ��γɵ����ʹ���ʵ��۵�ͷе����ߣ� |
| B�����д��������ˮ�в���������� |
| C��ÿһ��ˮ�����ں������������ |
| D��H2O��һ�ַdz��ȶ��Ļ������������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NO2��SO2��BF3����������ԭ�ӵ��������Ӷ�������8e���ȶ��ṹ |
| B��P4��CH4����������������Ҽ��Ƕ�Ϊ109o28�@ |
| C��CsCl��������ÿ��Cs+��������������Cl-����8�� |
| D��ԭ�Ӿ�����۷е�һ���Ƚ�������ĸ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com