
����Ŀ���������ʵ���A��B�����2L���ܱ������з�����Ӧ��3A(g)+B(g)xC(g)+D(g)����4min���D��Ũ��Ϊ0.4 mo1��L-1��C��ƽ����Ӧ����Ϊ0.lmo1��L-1��min-1��c(A)��c(B)=3��5������˵������ȷ����
A.x��ֵ��1B.4minĩ��A��ת����Ϊ60%
C.��ʼʱA��Ũ��Ϊ2.4mol��L-1D.4min��v(B)=0.1 mol��L-1��min-1

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����뻷֮�乲����������̼ԭ�ӵĶ������Ϊ�Ż����������ж���[1.1.0]���� (![]() )������һ�֡����й��ڸû������˵����ȷ����
)������һ�֡����й��ڸû������˵����ȷ����
A. ��C3H4��ͬϵ��
B. һ�ȴ���ֻ��һ��
C. �뻷��ϩ��Ϊͬ���칹��
D. ����̼ԭ�ӿ��ܶ�����ͬһƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���У���˵����ѧƽ��һ��������Ӧ�����ƶ����ǣ�������
A. N2O4(g)![]() 2NO2(g)���ı�ijһ��������������ɫ����
2NO2(g)���ı�ijһ��������������ɫ����
B. H2(g)+I2(g)![]() 2HI(g)����λʱ��������H2��HI�����ʵ���֮�ȴ���1:2
2HI(g)����λʱ��������H2��HI�����ʵ���֮�ȴ���1:2
C. N2(g)+3H2(g) ![]() 2NH3(g)���ı�ijһ������NH3�������������
2NH3(g)���ı�ijһ������NH3�������������
D. 2SO2(g) +O2(g)![]() 2SO3(g)�����º�ѹ�����£�����He
2SO3(g)�����º�ѹ�����£�����He
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ӧ2C��O2===2CO�������仯����ͼ��ʾ������˵����ȷ����(����)

A��12 g C(s)��һ����O2(g)��Ӧ����14 g CO(g)�ų�������Ϊ110.5 kJ
B���÷�Ӧ���Ȼ�ѧ����ʽ��2C(s)��O2(g)===2CO(g)����H����221 kJ
C��2 mol C(s)������O2(g)��Ӧ����CO2(g)���ų�����������221 kJ
D���÷�Ӧ�ķ�Ӧ�ȵ���CO�����л�ѧ���γ�ʱ���ͷŵ���������O2�����л�ѧ������ʱ�����յ��������IJ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NH4)2Cr2O7�������л��ϳɴ�����ýȾ������ӰҺ�ȡ�ij��ѧ��ȤС���(NH4)2Cr2O7�IJ������ʼ���ɽ���̽������֪��Cr2O72-(��ɫ��+H2O2CrO42-����ɫ��+2H+����ش��������⣺
��1�����Թ��м�������(NH4)2Cr2O7���壬�μ�����ŨKOH��Һ�����ȣ��۲쵽����Ҫ������___��
��2��Ϊ̽��(NH4)2Cr2O7(Ħ������Ϊ252g/mol)�ķֽ�������ͼ���Ӻ�װ�ã���A�м���5.040g��Ʒ����ʵ�顣
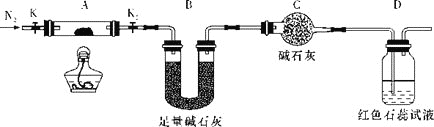
�����Ӻ�װ�ã���ȼ�ƾ���֮ǰ������еı�Ҫ������___��
�ڷ�Ӧ��������ȻҪͨһ��ʱ��ĵ�����ԭ����___��
�ۼ���A�����أ��۲쵽D����Һ����ɫ��ͬʱ���A�в�����ΪCr2O3��B�������ı仯Ϊ1.44g��д���ظ���識��ȷֽⷴӦ�Ļ�ѧ����ʽ��___��
��3��ʵ���ҳ��ü�ȩ���ⶨ��(NH4)2Cr2O7����Ʒ�е����������������ʲ�������Ӧ�����䷴Ӧԭ��Ϊ2Ba2++Cr2O72-+H2O=2BaCrO4��+2H+��4NH4++6HCHO=3H++6H2O+(CH2)6N4H+[�ζ�ʱ��1mo1(CH2)6N4H+��1mo1H+�൱]��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᡣ
ʵ�鲽�裺��ȡ��Ʒ5.600g�����500mL��Һ����ȡ25.00mL��Ʒ��Һ��250mL��ƿ�У����Ȼ�����ҺʹCr2O72-��ȫ��������10mL20.00mol��L��1�����Լ�ȩ��Һ��ҡ�ȡ�����5min����1��2�η�̪��Һ����0.200mo1L-1NaOH����Һ�ζ����յ㡣�ظ���������3�Σ����յζ���ȥNaOH����Һ�����ƽ��ֵΪ20.00mL��
�����ζ��յ�ʱ�����Ӷ�������ⶨ���___(����ƫ������ƫС��������Ӱ��������
�ڵζ�����ø���Ʒ��N����������Ϊ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����仯�����ڹ�ũҵ�����о�����Ҫ��;���ش��������⣺
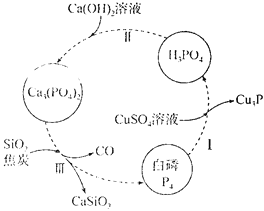
(1)��ͼ��ʾΪ�ᴿ������Ʒ(����������)�Ĺ������̡�����I�У�����ԭ��Ԫ����________(��Ԫ�ط���)������III�Ļ�ѧ����ʽΪ__________��
(2)���ᷰ�/̼���ϲ���[Li3V2(PO4)3/C]�dz��õĵ缫���ϣ����Ʊ��������£�
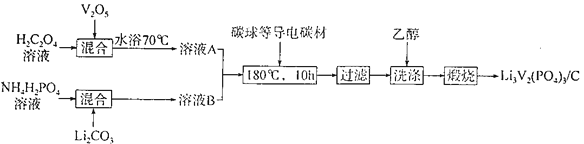
�ٸ��ϲ�����V�Ļ��ϼ�Ϊ________��C��������____________��
��V2O5��H2C2O4��Ӧ����V2(C2O4)3�Ļ�ѧ����ʽΪ____________����ϴ����ʱ���Ҵ�������ˮ��Ŀ����________________��
������ӵ����һ�ֶ��ε�أ��ֳ���ҡ������ء����ú�LixC6��Li3V2(PO4)3/C���缫���ŵ�ʱ�ĵ���ܷ�ӦΪLixC6��Li3��xV2(PO4)3= Li3V2(PO4)3+C6�����س��ʱ�����ĵ缫��ӦʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ����ࡢ��Ч����Դ�� Ҳ����Ҫ�Ļ���ԭ�ϡ�
(1)ͨ���Ȼ�ѧѭ���ڽϵ��¶���������ֽ��Ʊ������ķ�Ӧϵͳԭ��Ϊ��
SO2(g)+I2(s)+2H2O(l)=2HI(aq)+H2SO4(aq) H1=-151kJmol-1
2HI(aq)=H2(g)+I2(s) H2=+110kJmol-1
H2S(g)+H2SO4(aq)=S(s)+SO2(g)+2H2O(l) H3=+61kJmol-1
(�Ȼ�ѧ���ѭ������ֽ��������������ϵͳ)
ͨ�������֪����ϵͳ������Ȼ�ѧ����ʽΪ___________��
(2)��ҵ������CO��H2�ϳ������ԴCH3OH���䷴ӦΪCO(g)+2H2(g)CH3OH(g)��H= -116 kJ��mol-1����ͼ��ʾCO��ƽ��ת����(��)���¶Ⱥ�ѹǿ�仯��ʾ��ͼ��
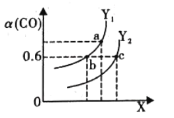
��X ��ʾ����______ (�����¶�������ѹǿ��) ��������_________��Y1______Y2 (����<������ >������=��)
����2L�����ܱ������г���2 mol CO ��4 mol H2��һ�������¾���10 min �ﵽƽ��״̬c �㴦���ڸ������£��ӿ�ʼ���ﵽƽ��״̬v(CH3OH) =______ molL-1min -1��ƽ�ⳣ��K=________(��������)��ƽ�ⳣ��Ka��Kb��Kc�Ĵ�С��ϵ��______
�����д�ʩ��������Ӧ����������߷�Ӧ��ת���ʵ���______ (����ĸ)��
A. ʹ�ô��� B. ��ʱ����CH3OH C.�����¶� D.����ѹǿ
(3) ��֪ȼ�ϵ�صı������뵥λ����ȼ������ʧȥ�ĵ����������ȡ�������H2��CH4��CH3OH�ļ��Ե�صı������ɴ�С��˳��Ϊ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ѧ�����
��1��������ʳ������Ӫ�����⣬��������ѧ�������ɳ�����Ҫ��֤��
������������������Ӫ�����ʣ��������������շֽ�Ϊ�� ����
A�������� B�������� C��֬����
������������������ Aʳ�� Bʳ�� Cƻ��֭ D������ E��ù�أ��밴����Ҫ�����(�����)��
����ά����C���� ����ֱ�ӽ���ѪҺ�������������� ��Ӧ����㷺�Ŀ�����֮һ���� ��������Ϊ��ζ�����ֿ���Ϊ���������� ��ʳ�ù��������Ѫѹ���ߡ���������� ��
��2����������������ͷ�չ�����ʻ���������ʹ�ò��Ͽ��Ը������ǵ����
������װ����ʹ�������Ļ��ͷų�һ�ֻӷ������ʣ����ڽӴ������������Ƥ�ף����߹����쳣���ûӷ��������ǣ� ����
A���ƾ� B������ C����ȩ
��һ������£��Ͻ��������ijɷֽ���Ӳ�� (���С)��
�����������뵼��������� ���ѧʽ��������ʳƷ����Ĥ�ĵĸ߷��Ӳ��ϵĽṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ��ˮ�ĵ���ﵽƽ�⣺H2O![]() H����OH��������������ȷ���ǣ� ��
H����OH��������������ȷ���ǣ� ��
A.����ˮ���ȵ�95��ʱ��Kw���pH���䣬ˮ�Գ�����
B.��ˮ�м���ϡ��ˮ��ƽ�������ƶ���c(OH-)����Kw��С
C.��ˮ�м�������Na2CO3���壬c(OH-)����Kw���䣬Ӱ��ˮ�ĵ���ƽ��
D.��ˮ�м������ᣬ������ˮ�ĵ��룻������ᣬ�ɴٽ�ˮ�ĵ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com