
����Ŀ��ij������ȤС����0.1000 mol��L1��NaOH����Һ�ζ�δ֪Ũ�ȵ�������Һ��ʵ��������£�������������⡣
A��___________________________��
B���ֱ�������ˮϴ�ɾ���ʽ�ζ��ܺͼ�ʽ�ζ��ܡ�
C���ô��ⶨ��������Һ��ϴ��ʽ�ζ��ܡ�
D������ʽ�ζ���ȡϡ���� 25.00 mL��ע������ϴ�ɾ�����ƿ�У�����ָʾ����
E����ʽ�ζ����ñ���NaOH��Һ��ϴ����Һע���ʽ�ζ��̶ܿ���0������2��3 cm�����ٰѼ�ʽ�ζ��̶ܹ��ã��ž����첿�ֵ����ݣ�������Һ�����̶���0������0���̶����¡�
F������ƿ���ڵζ������棬ƿ�µ�һ�Ű�ֽ���ߵα�ҡ����ƿֱ���ζ��յ㣬���µζ���Һ�����ڿ̶ȡ�
G����ȡ��ƿ�����ظ�����һ�Ρ�
��1�����ι���ʹ��ǰ����еIJ���A��___________________________��
��2���ζ�ʵ������IJ���������______________��������ĸ��
A����ʽ�ζ��� B����ʽ�ζ��� C����Ͳ D����ƿ
E������̨ F���ζ��ܼ� G���ձ� H����ֽ
��3����С��ͬѧѡ�÷�̪��ָʾ�����ζ��յ������Ϊ________________________________��
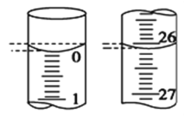
��4����С��ijһ�εζ������У���ʽ�ζ��ܵ�ʼ��Һ����ͼ��ʾ��
�ε�����������Ϊ ___________ mL��
��5����С��ѧ��ij3��ʵ����й����ݷֱ��¼���±���
�ζ����� | ����HCl��Һ�����/mL | 0.1000 mol/LNaOH�������mL�� | |
�ζ�ǰ�̶� | �ζ���̶� | ||
��һ�� | 25.00 | 2.00 | 27.91 |
�ڶ��� | 25.00 | 1.56 | 30.30 |
������ | 25.00 | 0.22 | 26.31 |
�����ϱ�������ʽ�����HCl��Һ�����ʵ���Ũ��Ϊ_______________��
��6�����в����п���ʹ�ⶨ���ƫ�͵���___________(����ĸ)��
A����ʽ�ζ���δ��ϴ��ֱ��ע�����ҺHCl��Һ
B���ζ�ǰʢ��HCl��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ��ܼ��첿���ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡNaOH��Һʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
���𰸡����ζ����Ƿ�©ˮ ABDG �����һ�α�Һ���룬��Һ����ɫ��Ϊdz��ɫ�Ұ�����ڲ��ָ� 26.10 0.1040 mol/L AD
��������
(1)�ζ���ʹ��ǰҪ����Ƿ�©ˮ��
(2)���ݲ�������ѡ��������
(3)��̪�ı�ɫ��ΧΪ��pHֵΪ8~10��С��8Ϊ��ɫ������10Ϊ��ɫ��
(4)�ζ�ǰ����Ϊ0.00mL���ζ������Ϊ26.10mL��
(5)����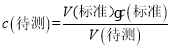 ���м��㣻
���м��㣻
(6)����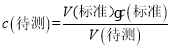 ������������
������������
(1)�ζ���ʹ��ǰҪ����Ƿ�©ˮ���ʴ�Ϊ�����ζ����Ƿ�©ˮ��
(2)����ҺΪ����ζ�ǰ��Ҫʢ�����ձ��У���Һʱ��Ҫ��ʽ�ζ��ܣ��ζ�ʱ��Ҫ��ƿʢ�ţ���ҺΪ����������Һ����Ҫ��ʽ�ζ��ܣ��ʴ�Ϊ��ABDG��
(3)��̪��pHֵΪ8~10ʱΪ����ɫ������10ʱΪ��ɫ����ҺΪ�������ƣ�����ҺΪ���ᣬ�����̪��ɫ�����������Ƶĵ�����Һ�����������ζ��յ���Һ�����ԣ��ʴ�Ϊ�������һ�α�Һ���룬��Һ����ɫ��Ϊdz��ɫ�Ұ�����ڲ��ָ���
(4)�ζ�ǰ����Ϊ0.00mL���ζ������Ϊ26.10mL����ʹ�õ��������Ϊ26.10mL���ʴ�Ϊ��26.10��
(5)��һ����������������Һ���Ϊ27.91mL-2.00mL=25.91mL���ڶ�����������������Һ���Ϊ30.30mL-1.56mL=28.74mL���ڶ�����������������Һ���Ϊ26.31mL-0.22mL=26.09mL���ڶ���ƫ��ϴ���ȥ���εζ���������������Һ���Ϊ��26.00mL�� 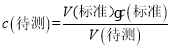 =
=![]() ���ʴ�Ϊ��0.1040 mol/L��
���ʴ�Ϊ��0.1040 mol/L��
(6)A����ʽ�ζ���δ��ϴ��ֱ��ע�����ҺHCl��Һ�Ὣ����Һϡ�ͣ����²��Ũ��ƫ�ͣ���A���ϣ�
B���ζ�ǰʢ��HCl��Һ����ƿ������ˮϴ����û�и��ﲻ��Բⶨ������Ӱ�죬��B�����ϣ�
C����ʽ�ζ��ܼ��첿���ڵζ�ǰ�����ݣ��ζ���������ʧ���ᵼ�±�Һ���ƫ�ⶨ���ƫ�ߣ���C�����ϣ�
D����ȡNaOH��Һʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ������ᵼ�±�Һ���ƫС����D���ϣ��ʴ�Ϊ��AD��


 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ��������������˵����ȷ���ǣ� ��
A.2L1mol/LNH4Cl��Һ��NH4+��Cl-��Ϊ2NA
B.�ڳ��³�ѹ�£�22g������̼���й��е��Ӷ�����Ϊ2NA
C.0.1molOH-����������0.1molH2O�ĵ�������ΪNA
D.�ڱ�״���£�11.2L�����е�̼ԭ����Ϊ4NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��101 kPaʱ��1molH2��ȫȼ������Һ̬ˮ���ų�285.8 kJ��������1molCH4��ȫȼ������Һ̬ˮ��CO2���ų�890.3 kJ�������������ܱ�ʾȼ���ȵ��Ȼ�ѧ����ʽ��
A.CH4(g)+2O2(g)![]() CO2(g)+2H2O(l) ��H=890.3 kJ
CO2(g)+2H2O(l) ��H=890.3 kJ
B.CH4(g)+2O2(g)![]() CO2(g)+2H2O(l) ��H=+890.3 kJ��mol1
CO2(g)+2H2O(l) ��H=+890.3 kJ��mol1
C.CH4(g)+2O2(g)![]() CO2(g)+2H2O(g) ��H=890.3 kJ��mol1
CO2(g)+2H2O(g) ��H=890.3 kJ��mol1
D.H2(g)+![]() O2(g)
O2(g)![]() H2O(l) ��H=285.8 kJ��mol1
H2O(l) ��H=285.8 kJ��mol1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ��ͼ��ʾ��װ�ý��е��ʵ�� ����˵����ȷ���� ( )
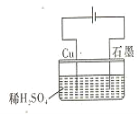
A.�������У�ͭ�缫����H2����
B.�����ڣ��ܷ�Ӧ����ʽΪ��Cu+H2SO4 ![]() CuSO4+H2
CuSO4+H2
C.���һ��ʱ���ʯī�缫����O2����
D.�����������У�H+��Ũ�Ȳ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ������淶���ܴﵽʵ��Ŀ�ĵ���
ѡ�� | ʵ��Ŀ�� | ���� |
A | ȡ20.00 mL���� | ��25 mL��ʽ�ζ�����װ�����ᡣ������ʼ����Ϊ5.00 mL��ʣ������ȫ��������ƿ�� |
B | ��������Na2CO3��Һ��pHֵ | ������ˮʪ��pH��ֽ������Na2CO3��Һ�У��۲�pH��ֽ��ɫ�������ɫ���Աȣ�����pHֵ |
C | ��ȡ������FeCl 3���� | ��������FeCl3��Һ |
D | ��֤Ksp[Cu(OH)2]�� Ksp[Mg(OH)2] | ��0.1 mol��L-1 MgSO4��Һ����NaOH��Һ�������г����������ٵμ�0.1 mol/L CuSO4��Һ |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ������Fe��Fe2O3�����Ͷ��250ml 2mol/L��HNO3��Һ�У���Ӧ��ȫ������ʣ�࣬����1.12L NO����(��״��)������Ӧ�����Һ�м���1mol/L��NaOH��Һ��Ҫʹ��Ԫ��ȫ������������������NaOH��Һ����������� ( )
A.450mlB.500ml
C.400mlD.���ж�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ճ�����������Ľ�����ij��ͬѧ��ѧϰ����֪ʶʱ�����������⣺
����1����Ϊ�γ�Ϊ��ɫ������
����2��CuO�ڸ����¿ɷֽ�Ϊ Cu2O��O2��Fe2O3�ڸ����¿ɷֽ�ΪFeO��O2��
(1)��������1��ͬѧ���������ң������ֽ��ͣ�
A����Ϊ�����������к�ɫ��������������Խк�ɫ����
B����Ϊ���ķ�ĩΪ��ɫ������������Ҳ��Ϊ��ɫ�����Խк�ɫ����
������Ϊ��ȷ��˵����__________��
������һ��ɫ��ĩ����μ��������ۣ�����Fe3O4��______________________________________��
������һ��ɫ��ĩ��Ϊ���������������Ļ������֤��������Fe3O4(ֻҪ�����ʵ�鷽��)��____________________________________��
(2)��������2��ͬѧ����ʵ��̽�����������������ַ�����
A���������������������գ�������ǰ����ɫ�Ƿ�仯��
B���������������������գ�������ǰ�������Ƿ�仯��
��ʵ����Ӧ��Fe2O3����__________(����������)�����ա�
������A�У�����������պ���ɫ��__________��Ϊ__________��˵��Fe2O3ȷʵ�����˱仯����˵�����ɵ�һ��ΪFeO��__________��������________________________��
������B�У����������Ԥ�ڵķ�Ӧ�������������ǰ��������ӦΪ________�����ǣ�ʵ������������ǰ��������Ϊ30��29����������պ���������____________________��
���Ƚ����ַ���������Ϊ�Ϻõķ�����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ������ȡCl2����Cl2Ϊԭ�Ͻ����ض���Ӧ��ʵ�飺

��1��AΪ��������װ�ã�д����Ӧ�Ļ�ѧ����ʽ_____________________.
��2��ʵ�鿪ʼ�ȵ�ȼA���ľƾ��ƣ�������K����Cl2��������װ�ã��ٵ�ȼD���ľƾ���.Cl2ͨ��Cƿ�����D��Dװ����ʢ��̼�ۣ�����������ԭ��Ӧ������CO2��HCl(��)����д��Dװ���з�Ӧ�Ļ�ѧ����ʽ_______________.װ��C��������________________.
��3��E��ʯ����Һ��������_____________����ԭ����________________.
��4������E����Һ��Ϊ����ʯ��ˮ����Ӧ���̵�������___________.
A���а�ɫ�������� B���ް�ɫ��������
C�������ɰ�ɫ������Ȼ�������ʧ
��5��D����Ӧ��Ϻر�����K����ȥ�ƾ��ƣ��������ȵ����ã�A����������Cl2��������ʱB�е�������________________________________________.B��������___________________________________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��C60�����ʯ��ʯī�Ľṹģ����ͼ��ʾ(ʯī����ʾ�����е�һ��ṹ)
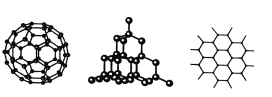
(1)C60�����ʯ��ʯī���ߵĹ�ϵ��Ϊ_________
A.ͬ���칹�� B.ͬ�������� C.ͬϵ�� D.ͬλ��
(2)��̬ʱ��C60����__________����(��������������ԭ��������������)��
(3)�辧��Ľṹ�����ʯ���ƣ�1 mol�辧���к��й�ĵ�������ĿԼ��________NA������������Ľṹ�൱���ڹ辧��ṹ��ÿ���赥��֮�����1����ԭ�ӡ���������Ŀռ���״�ṹ�У�����ԭ���γɵ���С������ԭ�ӵ���Ŀ��__________��
(4)ʯī��״�ṹ�У�ƽ��ÿ����������ռ�е�̼ԭ������__________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com