
����Ŀ���Թ�ҵ�����������Ϊԭ�Ͽ�����ȡ![]() ��������泥����Ʊ�����ͼ����,������������������գ�
��������泥����Ʊ�����ͼ����,������������������գ�
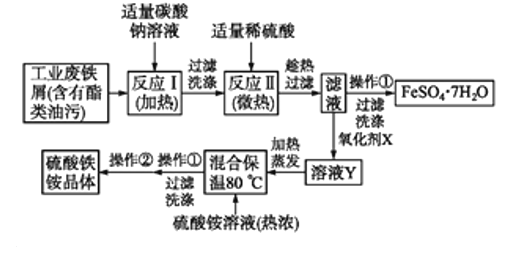
![]() ̼������Һ�ܳ�ȥ�������ۣ���ԭ����
̼������Һ�ܳ�ȥ�������ۣ���ԭ����![]() �����ӷ���ʽ��ʾ
�����ӷ���ʽ��ʾ![]() ______����Ӧ����Ҫ���������ӣ���ԭ����______��
______����Ӧ����Ҫ���������ӣ���ԭ����______��
![]() ����м�к����������������Ʊ�ǰ��ȥ����������
����м�к����������������Ʊ�ǰ��ȥ����������![]() �����ӷ���ʽ�ش�
�����ӷ���ʽ�ش�![]() ______���жϷ�Ӧ����ȫ��Ӧ��������______��
______���жϷ�Ӧ����ȫ��Ӧ��������______��
![]() �������������ʺϵ�������X��______��
�������������ʺϵ�������X��______��
![]()
![]()
![]()
![]()
![]() ����������ҺY֮ǰ����ȡ��������Һ������
����������ҺY֮ǰ����ȡ��������Һ������![]() �Ƿ���ȫ�����������������Լ�Ϊ______���ж��ܷ�������
�Ƿ���ȫ�����������������Լ�Ϊ______���ж��ܷ�������![]() ��Һ�����棬��˵��������______��
��Һ�����棬��˵��������______��
![]() ����
����![]() ��������______���������������
��������______���������������![]() �ķ�����______��
�ķ�����______��
![]() ��ȡ
��ȡ![]() �Ƶõ�������茶��壬��������ˮ���Ƴ�100mL��Һ���ֳ����ȷݣ�������һ����Һ�м�������NaOH��Һ�����ˡ�ϴ�ӡ������õ�
�Ƶõ�������茶��壬��������ˮ���Ƴ�100mL��Һ���ֳ����ȷݣ�������һ����Һ�м�������NaOH��Һ�����ˡ�ϴ�ӡ������õ�![]() ����������һ����Һ�м���
����������һ����Һ�м���![]() ��Һ��ǡ����ȫ��Ӧ�����������茶���Ļ�ѧʽΪ______��
��Һ��ǡ����ȫ��Ӧ�����������茶���Ļ�ѧʽΪ______��
���𰸡�![]() ���£��ٽ�ˮ�⣬��Һ������ǿ��ʹ��Ӧ��ֽ���
���£��ٽ�ˮ�⣬��Һ������ǿ��ʹ��Ӧ��ֽ��� ![]() �� ��м�����ܽ⣬��м���治�������ݲ�����
�� ��м�����ܽ⣬��м���治�������ݲ����� ![]() ���軯����Һ ���ܣ���Ϊ
���軯����Һ ���ܣ���Ϊ![]() ��
��![]() ����ʹ���Ե�
����ʹ���Ե�![]() ��Һ��ɫ ��ȴ�ᾧ ȡ������Ʒ�����Թ��У�������ˮ�ܽ⣬�ټ���NaOH��Һ���ȣ����Թܿ���ʪ��ĺ�ɫʯ����ֽ���飬������ֽ�����ɫ
��Һ��ɫ ��ȴ�ᾧ ȡ������Ʒ�����Թ��У�������ˮ�ܽ⣬�ټ���NaOH��Һ���ȣ����Թܿ���ʪ��ĺ�ɫʯ����ֽ���飬������ֽ�����ɫ ![]() ��
��![]()
��������
����м�к����������ۣ�![]() Ϊǿ�������Σ�
Ϊǿ�������Σ�![]() ˮ������
ˮ������![]() ��
��![]() �����´ٽ�
�����´ٽ�![]() ˮ�⣬��ٽ���֬ˮ�⣬Ȼ�����ϴ�ӣ�����ϡ���ᣬ������ӦIIΪ
ˮ�⣬��ٽ���֬ˮ�⣬Ȼ�����ϴ�ӣ�����ϡ���ᣬ������ӦIIΪ![]() �����ȹ��ˡ���ȴ�ᾧ������ϴ�ӵõ�
�����ȹ��ˡ���ȴ�ᾧ������ϴ�ӵõ�![]() ������������Һ���������������Ϊ��������Һ���������������ܼ���������Ũ���������Һ������
������������Һ���������������Ϊ��������Һ���������������ܼ���������Ũ���������Һ������![]() ��������������泥�����ȴ�ᾧ������ϴ�ӣ��������ɹ�ɵ�������������泥��ݴ˷������
��������������泥�����ȴ�ᾧ������ϴ�ӣ��������ɹ�ɵ�������������泥��ݴ˷������
![]() Ϊǿ�������Σ�
Ϊǿ�������Σ�![]() ˮ������
ˮ������![]() ��
��![]() ��ˮ�ⷽ��ʽΪ
��ˮ�ⷽ��ʽΪ![]() ����֬�ڼ���������ˮ�����ɿ�����ˮ�������κʹ����ε�ˮ��Ϊ���ȷ�Ӧ�����£��ٽ�ˮ�⣬��Һ������ǿ��ʹ��Ӧ��ֽ��У��ʴ�Ϊ��
����֬�ڼ���������ˮ�����ɿ�����ˮ�������κʹ����ε�ˮ��Ϊ���ȷ�Ӧ�����£��ٽ�ˮ�⣬��Һ������ǿ��ʹ��Ӧ��ֽ��У��ʴ�Ϊ��![]() �����£��ٽ�ˮ�⣬��Һ������ǿ��ʹ��Ӧ��ֽ��У�
�����£��ٽ�ˮ�⣬��Һ������ǿ��ʹ��Ӧ��ֽ��У�
![]() ����м�к����������������Ʊ�ǰ��ȥ����Ϊ���������ᷴӦ�������Σ������ܱ�Fe��ԭ���������Σ��漰�����ӷ���ʽΪ
����м�к����������������Ʊ�ǰ��ȥ����Ϊ���������ᷴӦ�������Σ������ܱ�Fe��ԭ���������Σ��漰�����ӷ���ʽΪ![]() ��
��![]() ����м�����ܽ⣬��м���治�������ݲ�������Ӧ����ȫ��Ӧ���ʴ�Ϊ��
����м�����ܽ⣬��м���治�������ݲ�������Ӧ����ȫ��Ӧ���ʴ�Ϊ��![]() ��
��![]() ����м�����ܽ⣬��м���治�������ݲ�����
����м�����ܽ⣬��м���治�������ݲ�����
![]() ������X�����������ӣ�ͬʱ�����������ʣ���ѡ��������⣻�ʴ�Ϊ��b��
������X�����������ӣ�ͬʱ�����������ʣ���ѡ��������⣻�ʴ�Ϊ��b��
![]() �������������軯�ؼ��飬��������ʱ����Ĺ��������������������Ҳ��ʹ���Ը��������ɫ���ʲ�ѡ���Ը�����أ��ʴ�Ϊ�����軯����Һ�����ܣ���Ϊ
�������������軯�ؼ��飬��������ʱ����Ĺ��������������������Ҳ��ʹ���Ը��������ɫ���ʲ�ѡ���Ը�����أ��ʴ�Ϊ�����軯����Һ�����ܣ���Ϊ![]() ��
��![]() ����ʹ���Ե�
����ʹ���Ե�![]() ��Һ��ɫ��
��Һ��ɫ��
![]() ����������Һ���ȹ��˺���ȴ�ᾧ������ϴ�ӣ��õ������������������壬����
����������Һ���ȹ��˺���ȴ�ᾧ������ϴ�ӣ��õ������������������壬����![]() ��ȴ�ᾧ������笠����ӵķ���Ϊ��ȡ������Ʒ�����Թ��У�������ˮ�ܽ⣬�ټ���NaOH��Һ���ȣ����Թܿ���ʪ��ĺ�ɫʯ����ֽ���飬������ֽ�����ɫ���ʴ�Ϊ����ȴ�ᾧ��ȡ������Ʒ�����Թ��У�������ˮ�ܽ⣬�ټ���NaOH��Һ���ȣ����Թܿ���ʪ��ĺ�ɫʯ����ֽ���飬������ֽ�����ɫ��
��ȴ�ᾧ������笠����ӵķ���Ϊ��ȡ������Ʒ�����Թ��У�������ˮ�ܽ⣬�ټ���NaOH��Һ���ȣ����Թܿ���ʪ��ĺ�ɫʯ����ֽ���飬������ֽ�����ɫ���ʴ�Ϊ����ȴ�ᾧ��ȡ������Ʒ�����Թ��У�������ˮ�ܽ⣬�ټ���NaOH��Һ���ȣ����Թܿ���ʪ��ĺ�ɫʯ����ֽ���飬������ֽ�����ɫ��
![]() ��ȡ
��ȡ![]() ��Ʒ����������ˮ���Ƴ�100mL��Һ���ֳ����ȷݣ�������һ���м�������NaOH��Һ������ϴ�ӵõ�
��Ʒ����������ˮ���Ƴ�100mL��Һ���ֳ����ȷݣ�������һ���м�������NaOH��Һ������ϴ�ӵõ�![]() ������ӦΪ
������ӦΪ![]() ��
��![]() ������һ����Һ�м���
������һ����Һ�м���![]() ��Һ��ǡ����ȫ��Ӧ����
��Һ��ǡ����ȫ��Ӧ����![]() ������
������![]() ��Ʒ�к���
��Ʒ�к���![]() ��
��![]() Ϊ
Ϊ![]() ����
����![]() Ϊ
Ϊ![]() ����
����![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��2��2�����Ի�ѧʽΪ
��2��2�����Ի�ѧʽΪ![]() ��
��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼװ��̽��NH3��CuSO4��Һ�ķ�Ӧ��
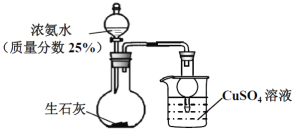
(1)�����Ʊ�NH3��ʵ���У���ƿ�з�Ӧ�漰�����ƽ����ƶ���NH3+H2O![]() NH3H2O��____________��_________________(���о����е�����ƽ�⣬��д��ѧ����Ҳ�����ֱ���)��
NH3H2O��____________��_________________(���о����е�����ƽ�⣬��д��ѧ����Ҳ�����ֱ���)��
(2) �Ʊ�100mL25%��ˮ(��=0.905gcm-3)����������Ҫ��״���°���______L(С�������һλ)��
(3) ����ʵ�鿪ʼ���ձ��ڵ���Һ__________________________�����ﵽ��ֹ������Ŀ�ġ�
(4)NH3ͨ��CuSO4��Һ�У�������ɫ������д���÷�Ӧ�����ӷ���ʽ��_______________________������ͨ������������������ʧ�õ�����ɫ[Cu(NH3)4]2+��Һ���������·�Ӧ��2NH4+(aq)+Cu(OH)2(s)+2NH3(aq)![]() [Cu(NH3)4]2+(aq)(ͭ����Һ)+2H2O+Q(Q>0)��
[Cu(NH3)4]2+(aq)(ͭ����Һ)+2H2O+Q(Q>0)��
�ٸ÷�Ӧƽ�ⳣ���ı���ʽK=___________________________��
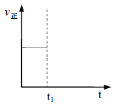
��t1ʱ�ı�������һ��ʱ���ﵽ��ƽ�⣬��ʱ��ӦK��������ͼ�л����ù�����v���ı仯___________________��
��������ͭ����Һ�м�ˮϡ�ͣ�������ɫ������ԭ���ǣ�________________________________��
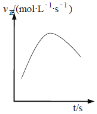
���ھ����ܱ������У�����NH4+(aq)��Cu(OH)2��NH3(aq)����������Ӧ��v����ʱ��ı仯����ͼ��ʾ��v����������С��ԭ��__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͬ��ͬѹ�£���3֧��ͬ������Թ��зֱ���е������ϵ��������壬�����Ǣ�NO��NO2����NO2��O2����NO��N2���ֽ�3֧�Թܾ�������ˮ���У���ַ�Ӧ���Թ���ʣ����������ֱ�ΪV1��V2��V3�������й�ϵ��ȷ����(����)
A.V1>V2>V3B.V1>V3>V2
C.V2>V3>V1D.V3>V1>V2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ�ֹ��ɽ���Ԫ�أ�ͨ�������Ͻ���ֵ����Ӽ�������Ԫ�ؿ���ǿ�Ͻ��ǿ�ȡ�Ӳ�ȡ��ɺ����Լ����ԣ�������ǿ�����¼���ʴ���ܡ���ͼ�ǻ����������Ʊ����������Ҫ����ͼ��
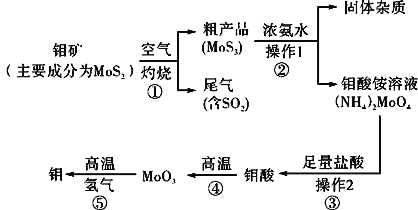
��1����Ӧ�ٵ�β�����������ã�д��Ӧ�ø�β���Ƶõ�������Ҫ��ѧ�Լ���___��
��2�������ʵ����ģ�����1�Ͳ���2������Ҫʹ�õ���Ҫ���������У�___��
��3�����ڿ��������������������⣬����������������������Һ���������ƣ��������ⲻ���������ϡ���ᡣ�����ƵĻ�ѧʽΪ___��
��4����ҵ���Ʊ���ԭ������CO��H2�ķ�Ӧԭ��ΪCO2��CH4![]() 2CO��2H2��CH4��H2O
2CO��2H2��CH4��H2O![]() CO��3H2���������������Ϊ80%��a L(��״��)��Ȼ��������������̼��ˮ�����Ļ�����ڸ����·�Ӧ������ת����Ϊ90%���ò����Ļ�ԭ������(CO��H2)��ԭMoO3���⣬�������������������Ϊ___��
CO��3H2���������������Ϊ80%��a L(��״��)��Ȼ��������������̼��ˮ�����Ļ�����ڸ����·�Ӧ������ת����Ϊ90%���ò����Ļ�ԭ������(CO��H2)��ԭMoO3���⣬�������������������Ϊ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������У���Ϊͬ���칹����� (����)
A. ��
��
B. ��CH3CH2CH2CH2Cl
��CH3CH2CH2CH2Cl
C.CH4��CH3CH3
D.CH3CH(CH3)CH2CH2CH3��CH3CH2CH2CH(CH3)CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��ʾҳ������ijЩ����Ľṹģ�ͣ�

(1)�л��������Ϊ____________���л�������л����һ�Ϊ____________��
(2)�л����ҵ�һ��ȡ������________�֣�������ݼ�����ӽṹʾ��ͼ�Ʋ⣬�л������������________��Cԭ����ͬһƽ���ڡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼��������������ӵ�ص��µ��Һ����Ҫ���Ӽ�����ṹ��ͼ��![]() �������й�˵������ȷ����
�������й�˵������ȷ����
A.����ʽΪC3H4O3B.��������������������֮��Ϊ3:1
C.�����м��м��Լ�Ҳ�зǼ��Լ�D.������̼ԭ�ӵ��ӻ���ʽȫ��Ϊsp2�ӻ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��С���Ʊ��������(K2FeO4) ��̽�������ʡ�����: K2FeO4 Ϊ��ɫ���壬����KOH��Һ;����ǿ�����ԣ������Ի�������Һ�п��ٲ���O2,�ڼ�����Һ�н��ȶ����Ʊ�K2FeO4 (�г�װ����)
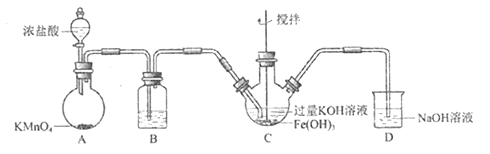
��1�����������װ�������Եķ�����________________________________________��
��2��AΪ��������װ�á�A�л�ѧ��Ӧ�ı���ԭ��Ԫ����____________________________��
��3��װ��B�������dz��ӣ������Լ�Ϊ_____________________________________��
��4��C�еõ���ɫ�������Һ����д��C�з����Ļ�ѧ��Ӧ���������ת�Ƶķ������Ŀ��_________�� �˷�Ӧ����:������Cl2______FeO42-(����>������<��)��
��5�� C�г��˷������еķ�Ӧ����������ѧ��Ӧ�����ӷ���ʽ��:______________________��
��6����KOH��Һ���ϴ��C�����ù��壬����KOH��Һ��K2FeO4�ܳ����õ���ɫ��Һa��ȡ����a,�μ����ᣬ��Cl2��������ʵ��ó�Cl2��FeO42-��������ǿ����ϵ���Ʊ�ʵ��ʱ�ó��Ľ����෴��ԭ����__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W��Ϊ������Ԫ�أ�������Ԫ�����ڱ������λ����ͼ��ʾ����Zԭ�ӵ������������ǵ�һ���������3��������˵����ȷ���ǣ�������
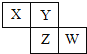
A.Z������������Ӧ��Y����
B.X��W��ԭ�Ӻ�����������9
C.X����������ǿ��Y����
D.����������Ӧˮ��������W��Zǿ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com