
����Ŀ����ǹ�ҵ���Ʊ���������ƾ��壨Na2S2O35H2O���ķ���֮һ���������£�
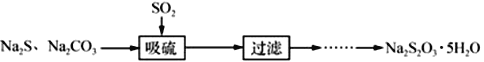
��֪��Na2S2O3�ڿ�����ǿ�Ȼᱻ������Na2S2O35H2O��M=248g/moL����35 �����ϵĸ����������ʧȥ�ᾧˮ����������Ӱ������ԭ����ij��ȤС����ʵ��������Ʊ�Na2S2O35H2O��̽��Na2S2O3�Ļ�ѧ���ʡ�
I���Ʊ�Na2S2O35H2O
�����������װ�ã�
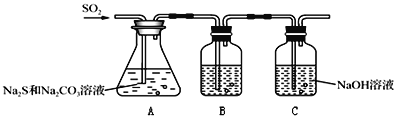
��1��д��Aƿ������Na2S2O3��CO2�����ӷ���ʽ______��
��2��װ��B�������Ǽ���װ��A��SO2������Ч����װ��B���Լ�������______
A Ũ���� B ��ˮ C FeSO4��Һ D BaCl2��Һ
II���ⶨ��Ʒ����
��1��Na2S2O3��Һ�Ƕ���ʵ���еij����Լ����ⶨ��Ũ�ȵĹ������£�
��һ����ȷ��ȡa gKIO3��M=214g/moL�����������Һ��
�ڶ������������KI��H2SO4��Һ���μ�ָʾ����
����������Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��Һ�����ΪV mL��
��c��Na2S2O3����______ mol /L�����г���ʽ���ɣ�����֪��IO3��+5I��+6H+��3I2+3H2O��2S2O32��+I2��S4O62��+2I����
��2���ζ�����������ʵ���������ɽ��ƫ�ߵ���_________������ĸ��
A �ζ���δ��Na2S2O3��Һ��ϴ
B �ζ��յ�ʱ���Ӷ���
C ��ƿ������ˮ��ϴ��δ�ô�ȡҺ��ϴ
D �ζ��ܼ��촦�ζ�ǰ�����ݣ���ζ��յ�ʱδ����������
��̽��Na2S2O3�Ļ�ѧ����
��֪Na2S2O3��Һ��Cl2��Ӧʱ��1mol Na2S2O3ת��8mol���ӡ���ͬѧ�����ͼʵ�����̣�
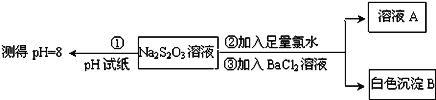
��1����ͬѧ���ʵ�����̵�Ŀ����֤��Na2S2O3��Һ����___________��__________��
��2����ͬѧ��ΪӦ�����������Тڢ������Լ�˳��ߵ�������Ϊ������__________��
���𰸡�2S2��+ CO32��+ 4SO2 ��3S2O32��+ CO2 B ![]() ����
����![]() �� B ���� ��ԭ�� �����ų�BaS2O3�ĸ���
�� B ���� ��ԭ�� �����ų�BaS2O3�ĸ���
��������
I��(1)����ͼʾ��Ϣ��֪������װ��A��ȡNa2S2O3�ķ�Ӧ��ΪSO2��Na2S��Na2CO3��������ΪNa2S2O3�����ݵ�ʧ�������غ�ó�SO2��Na2S��Na2S2O3�ļ��������ٸ��������غ�ó�Na2CO3�ļ���������һ�ֲ���CO2���ݴ˷�����
(2)����������л�ԭ�ԡ�Ư���ԣ�
II��(1)����KIO3�������I2���ٸ���S2O32-��I2�Ĺ�ϵ���Na2S2O3�����ʵ�����Ũ�ȣ�
(2)�ζ�ʱ����������������c(��)V(��)=c(��)V(��)��c(��)= ������
������
����(1) ��ͬѧ��ʵ��������ͨ������BaCl2 ������ɫ����B ��֤��Na2S2O3 ����ˮ��Ӧʱ��SO42-���ɣ�
(2) ��֤��Na2S2O3�Ļ�ԭ��ʱ���ڲ�֪��BaS2O3 �Ƿ��dz���������Ӧ�ȼ�BaCl2 ��Һ�������������ɫ�����ټ�������ˮ������ɫ����������֤��Na2S2O3���л�ԭ�ԡ�
I��(1) ����ͼʾ��Ϣ��֪������װ��A��ȡNa2S2O3�ķ�Ӧ��ΪSO2��Na2S��Na2CO3��������ΪNa2S2O3��SO2��Na2S����Ԫ����+4�ۺ�2�۱�Ϊ+2�ۣ����ݵ�ʧ�������غ�ó�SO2��Na2S��Na2S2O3�ļ������ֱ�Ϊ4��2��3���ٸ��������غ�ó�Na2CO3�ļ�����Ϊ1������̼ԭ�Ӻ���ԭ�����غ��֪��һ�ֲ���CO2���Ҽ�����Ϊ1���ʷ���ʽΪ��2S2��+ CO32��+ 4SO2��3S2O32��+ CO2��
(2)����������л�ԭ�ԡ�Ư���ԣ����Կ�����Ʒ�졢��ˮ��![]() ��Һ����������������Ƿ���ȫ���գ���SO2����Ч�ʵͣ������������ʣ�࣬B�е���Һ����ɫ��
��Һ����������������Ƿ���ȫ���գ���SO2����Ч�ʵͣ������������ʣ�࣬B�е���Һ����ɫ��
II��(1) KIO3+5KI+3H2SO4=3K2SO4+3I2+3H2O��I2+2Na2S2O3=Na2S4O6+2NaI��
n(KIO3)=![]() mol����μӷ�Ӧ��Na2S2O3Ϊxmol��
mol����μӷ�Ӧ��Na2S2O3Ϊxmol��
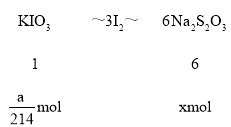
����x=![]() ����c(Na2S2O3)=
����c(Na2S2O3)=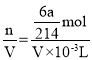 =
=![]() molL1����
molL1����![]() ��molL1��
��molL1��
(2) A.�ζ���ĩ��Na2S2O3��Һ��ϴ����Na2S2O3��Һ�ᱻϡ�ͣ��ζ�ʱ���Ĵ���Һ���ƫ���´���ƫ�ͣ���A���������⣻
B. �ζ��յ�ʱ���Ӷ�����ʹNa2S2O3��Һ���ƫС���ζ�ʱ���Ĵ���Һ���ƫС�����´���ƫ�ߣ���B�������⣻
C.��ƿ������ˮ��ϴ����ʵ����ûӰ�죬���Ȳ��䣬��C���������⣻
D. �ζ��ܼ��촦�ζ�ǰ�����ݣ���ζ��յ�ʱδ���������ݣ�����Һ���ƫ������Ʒ����ƫ�ͣ���D���������⣻
�ʴ�ѡB��
����(1)��ͬѧͨ���ⶨNa2S2O3��Һ��pH=8��˵�����ε�ˮ��Һ�Լ��ԣ���ͬѧ��ʵ��������ͨ������BaCl2������ɫ����B��֤��Na2S2O3����ˮ��Ӧʱ��SO42����,��֤��S2O32���л�ԭ�ԣ�
(2)��֤��Na2S2O3�Ļ�ԭ��ʱ���ڲ�֪��BaS2O3�Ƿ��dz���������Ӧ�ȼ�BaCl2��Һ�������������ɫ�����ټ�������ˮ������ɫ����������֤��Na2S2O3���л�ԭ�ԣ����ҿ��ų�BaS2O3�ĸ��š�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����10 mL 0.l mol��L��1 H2C2O4��Һ�еμӵ�Ũ�ȵ�NaOH��Һ����Һ��pH��NaOH��Һ�������ϵ��ͼ��ʾ������������ȷ����( )
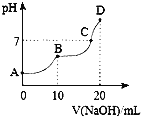
A.A����Һ�У�c(H��)��c(OH��)��c(HC2O4��)��2c(C2O42��)
B.HC2O4������Һ��ˮ��̶ȴ��ڵ���̶�
C.C����Һ�к��д���NaHC2O4��H2C2O4
D.D����Һ�У�c(Na��)��c(C2O42��)��c(HC2O4��)��c(OH��)��c(H��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NH3���������з�ȼ�ϵ���ǵ�ǰ���е�һ���ȵ㡣ʹ�õĵ������Һ��2mol��L1��KOH��Һ������ܷ�ӦΪ��4NH3+3O2��2N2+6H2O���õ�ظ����ĵ缫��ӦʽΪ____________________��ÿ����3.4g NH3ת�Ƶĵ�����ĿΪ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶��£���10 mL 0.1 mol��L��1 CuCl2��Һ�еμ�0.1 mol��L��1��Na2S��Һ���μӹ����У�lg c(Cu2��)��Na2S��Һ����Ĺ�ϵ��ͼ��ʾ�������й�˵����ȷ����
��֪��Ksp(ZnS)��3��10��25
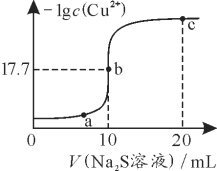
A. Na2S��Һ�У�c(S2��)��c(HS��)��c(H2S)��2c(Na��)
B. a��b��c�����Ӧ����Һ�У�ˮ�ĵ���̶�����Ϊb��
C. ���¶��£�Ksp(CuS)��1��10��35.4
D. ��100 mL Zn2����Cu2�����ʵ���Ũ�Ⱦ�Ϊ0.1mol��L��1�Ļ����Һ����μ���10��3 mol��L��1��Na2S��Һ��Zn2���ȳ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ܱ�������һ����������巢����Ӧ��x A(g)��y B(g) ![]() z C(g)ƽ��ʱ���A��Ũ��Ϊ0.5 mol/L�������¶Ȳ��䣬���������ݻ�����ԭ����2�����ٴ�ƽ��ʱ���A��Ũ��Ϊ0.2 mol/L�������ж���ȷ����
z C(g)ƽ��ʱ���A��Ũ��Ϊ0.5 mol/L�������¶Ȳ��䣬���������ݻ�����ԭ����2�����ٴ�ƽ��ʱ���A��Ũ��Ϊ0.2 mol/L�������ж���ȷ����
A. ƽ�����淴Ӧ�����ƶ�B. x��y<z
C. C������������ֲ���D. B��ת���ʽ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾװ��(������Ϊ���Ե缫)������SO2�����������ų�����Һ����NO2�����й��ڸ�װ��������˵������ȷ�������

��.aΪֱ����Դ�ĸ���
��.�����ĵ缫��ӦʽΪ��2HSO3-+2H++2e-=S2O42-+2H2O
��.�����ĵ缫��ӦʽΪ��SO2+2H2O-2e-=SO42-+4H+
��.���ʱ��H+��������ͨ�������ӽ���Ĥ��������
A. ������ B. ������
C. �ں͢� D. �ۺ͢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ӧ�����������Ϊ��̬��ij���淴Ӧ�ڲ�ͬ�����µķ�Ӧ���̷ֱ�ΪA��B����ͼ��ʾ��

��1����ͼ�жϸ÷�Ӧ��______(����������������)�ȷ�Ӧ������Ӧ�� ��ƽ��������������䣬�����¶ȣ���Ӧ���ת����____(����������������С������������)
��2������B���̱����˷�Ӧ���õ�����Ϊ___(ѡ�����������ĸ),������ȷ���жϵ�����Ϊ________
A�������¶� B������Ӧ���Ũ�� C�������¶� D��ʹ���˴���
��3������H����ֵΪ200kJ/mol����xֵӦΪ__kJ/mol.�˷�Ӧ��A��Ӧ�����е�����Ӧ�Ļ��Ϊ _____kJ/mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ��ʯ�ͻ�������Ҫԭ�ϣ�һ�������¿ɷ�������ת����

��֪��![]()
��1��A�Ľṹ��ʽΪ��____________��
��2�� ��Ӧ�ܵ�����Ϊ��_________________��Ӧ����Ӧ�߽�������������ǣ�_________��
��3��D�������Ҵ���Ӧ����E�Ļ�ѧ����ʽΪ��__________________��
��4�� ������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ_________________��
������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ_________________��
��5��B��ͬ���칹���ж��֣�д������һ�ּ��ܷ���������Ӧ�����ܷ���������Ӧ,���Һ˴Ź�������ֵΪ6��1��1��ͬ���칹��Ľṹ��ʽ��_____________________��
��6����д���Ա�ϩΪԭ���Ʊ���2-�ǻ�����ĺϳ�·�ߣ����Լ���ѡ��______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ȼ���ͭ�ǻ�����ӡȾ����ҵ����Ҫԭ�ϣ��㷺�����л��ϳɵĴ�����
��.�ײ���CuCl2��2H2O�ȷֽⷨ�Ʊ�CuCl��װ����ͼ��

��1������X��������_______________��C���ձ����Լ�������__________��
��2�������������ͨ���������_______��
��3����Ӧ������ȡ���ò�Ʒ�������������к�������ͭ�������ԭ����______________��
��.����ȡ����CuCl2���������·����Ʊ�CuCl��

��4���������з�Ӧ�����ӷ���ʽΪ___________________��
��5��������������100 mL 10 mol/L�������0.2 mol/L���ᣬ��ͨ��SO2���ް�ɫ�����������Դ��������������ֲ��룺
����һ��c(H��)�����°�ɫ�����ܽ⡣Ϊ��֤�˲��룬ȡ75gCuCl2���塢100 mL0.2 mol/L���ἰ________mL10.0mol/LH2SO4���Ƴ�200 mL��Һ���ٽ��в����ڣ��۲��Ƿ��а�ɫ����������
�������_______________�������ʵ��˵���ò����Ƿ������_________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com