
����Ŀ����1����16gO2����ԭ��������ͬ��NH3��״���������________L��
��2����֪2L Al2(SO4)3��Һ��c(Al3+)=3mol/L������3L__________mol/LNa2SO4��SO42�������ʵ���Ũ����ȡ�
��3��ͬ��ͬѹ�£�ͬ�����NH3��H2S�����������Ϊ_____________��ͬ������NH3��H2S����������Ϊ___________�����к��е����ԭ�Ӹ�����Ϊ________����NH3��H2S�е���ԭ������ȣ����ǵ������Ϊ____________��
��4���ڱ�״���£�8.96L��CH4��CO�Ļ�����壬����������������ܶ���9.5������������CH4�����Ϊ____________��CH4��COԭ�Ӹ�����Ϊ_______________��
���𰸡�5.6 4.5 1��2 2��1 3��1 2��3 6.72L 15��2
��������
��1������ԭ���������Ϊ�������㣻
��2������c(SO42-)Ϊ�������㣻
��3�����ð����ӵ����ɼ������۽��
��4�����ð����ӵ���������![]() ���
���
��1��16gO2�����ʵ���Ϊ��16g��32gmol-1=0.5mol������ԭ������ΪNA��һ��NH3���Ӻ�4��ԭ�ӣ����Ժ�ԭ������ΪNA��NH3����Ϊ![]() NA������0.25mol���ڱ�״��������ǣ�0.25mol��22.4Lmol-1=5.6L��
NA������0.25mol���ڱ�״��������ǣ�0.25mol��22.4Lmol-1=5.6L��
��2��Al2(SO4)3��Һ��c(Al3+)=3mol/L����c(SO42-)=![]() ��3mol/L=4.5mol/L����Na2SO4��Һ��SO42-�����ʵ���Ũ��Ϊ4.5mol/L����Na2SO4��Һ�����ʵ����ʵ���Ũ��Ϊ4.5mol/L��
��3mol/L=4.5mol/L����Na2SO4��Һ��SO42-�����ʵ���Ũ��Ϊ4.5mol/L����Na2SO4��Һ�����ʵ����ʵ���Ũ��Ϊ4.5mol/L��
��3��ͬ��ͬѹ�£�ͬ�����NH3��H2S�������ʵ�����ȣ���m=nM�ɵ������ȵ���Ħ������֮�ȣ����������=17��34=1��2��ͬ������NH3��H2S���ʵ���֮����Ħ������֮�ȳɷ���=2��1�����֮��=���ʵ���֮��=2��1����Ϊͬ������NH3��H2S���ʵ���֮��=2��1��һ��NH3�����к�Hԭ�Ӹ���Ϊ3��һ��H2S�����к�Hԭ�Ӹ���Ϊ2������ͬ������NH3��H2S������������ԭ�Ӹ�����=(2��3)��(1��2)=3��1����Ϊһ��NH3��H2S����������H���ֱ�Ϊ3��2��������ԭ����Ŀ���ʱ��NH3��H2S�����ʵ���֮��Ϊ2��3��ͬ��ͬѹ�£������Ϊ2��3��
��4���ڱ�״���£�CH4��CO�Ļ���������������ܶ���9.5����������ƽ����Է�������Ϊ9.5��2=19����ƽ��Ħ������Ϊ19g/mol����CH4��CO�����ʵ���֮��Ϊx��y����16x+28y=19(x+y)�����x��y=3��1����Ϊ��ͬ״��������ȵ������ʵ���֮�ȣ����Ի��������CH4�����Ϊ��8.96L��![]() =6.72L����״����8.96L�����������ʵ���Ϊ��8.96L��22.4Lmol-1=0.4mol��CH4��CO�����ʵ���֮��Ϊ3��1����CH4�����ʵ���Ϊ0.3mol��CO�����ʵ���Ϊ0.1mol������ԭ�Ӹ�����Ϊ��(3��5)��(1��2)=15��2��
=6.72L����״����8.96L�����������ʵ���Ϊ��8.96L��22.4Lmol-1=0.4mol��CH4��CO�����ʵ���֮��Ϊ3��1����CH4�����ʵ���Ϊ0.3mol��CO�����ʵ���Ϊ0.1mol������ԭ�Ӹ�����Ϊ��(3��5)��(1��2)=15��2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��д�������ĸ���Ӧ�ķ���ʽ��
(1)����ˮ�����ķ�Ӧ___________��д����ѧ����ʽ����
(2)�������ƺͶ�����̼�ķ�Ӧ___________��д����ѧ����ʽ����
(3)��������������Һ�ķ�Ӧ___________��д�����ӷ���ʽ����
(4)���������������Ʒ�Ӧ_____________��д�����ӷ���ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й��������ʵ�˵����ȷ���ǣ� ��
A. ʯ��ͨ����ѹ������Եõ���Է������ϴ�ķ�����
B. ��ϩ��������Ȼ�̼��Һ��Ӧ����1,3-�������
C. ![]() ��������16��ԭ�ӹ�ƽ��
��������16��ԭ�ӹ�ƽ��
D. ![]() ��һ�ȴ����ͬ���칹����12��
��һ�ȴ����ͬ���칹����12��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA���������ӵ�������ֵ������˵����ȷ���ǣ� ��
A. ���³�ѹ�£�11.2 L������������ԭ����ΪNA
B. 46g NO2��46g N2O4���е�ԭ������Ϊ3NA
C. lmolO2��Ϊ�������õ��ĵ�����һ��Ϊ4NA
D. 1.8 g D2O���е�����������������ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijС��Ϊ�о��绯ѧԭ���������ͼװ�ã�������������ȷ���ǣ� ��
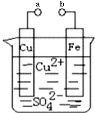
A. a��b������ʱ����Ƭ�ϻ��н���ͭ����
B. a��b�õ�������ʱ����Ƭ�Ϸ����ķ�ӦΪ��Fe��2e��=Fe2��
C. a��b�õ�������ʱ���Ӵ�CuƬ������Ƭ
D. a��b�õ�������ʱ��Cu2����ͭ�缫�ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ᣨH2C2O4����һ�ֶ�Ԫ���ᣬ�ڻ�ѧ���������Ҫ����;��ijʵ��Ա��Ҫ�ò��ᾧ�壨H2C2O42H2O������400mL 0.4mol/L��H2C2O4��Һ����ش���������
��1��ͨ�����㣬��ʵ��ԱӦ����������ƽ��______ g H2C2O42H2O������ʵ�顣
��2����д����ʵ���ʵ�鲽��
a:���㣬b������c:_____��d:��ȴ,e:��Һf:ϴ��g:________��h:ҡ�ȣ�i:װҺ��
��3���ڴι������õ��IJ����������˲��������ձ�����ͷ�ι��⣬����_____��
��4���������ƹ����У�����ʵ������������Ƶ���Һ�����ʵ���Ũ���к�Ӱ�죿��ѡ�ƫ�ߡ���ƫ�͡�����Ӱ�족����
I��������ĩʱ���������λ�÷ŷ�����___________��
II����Һ���̺�û�жԲ��������ձ�����ϴ����_________��
III.����ʱ���ӿ̶�����___________��
IV.ҡ��ʱ������ƿҺ����ڿ̶��ߣ��μ�����ˮ���̶�����_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ������У���ȷ����( )
|
|
|
|
A.����������Һʱ����Ũ��������ע��ʢ��ˮ������ƿ�н������� | B.��������ƽ����ҩƷʱ����ҩƷ������ƽ���� | C.�����Һ�������ʱ��Ӧʹ�¶ȼ�ˮ�����û�ڻ��Һ�� | D.��Һʱ�������²�Һ�����δӷ�Һ©���¿ڷֱ����������ձ��� |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ҵ��ˮ�����±��е�ijЩ���ӣ��Ҹ������ӵ����ʵ���Ũ����ȣ���Ϊ0.1 mol/L(����ֵ����ˮ�ĵ��뼰���ӵ�ˮ��)��
������ | K����Ag����Mg2����Cu2����Al3����NH4+ |
������ | Cl����CO32����NO3����SO42����SiO32����I�� |
��ͬѧ��̽����ˮ����ɣ�����������ʵ�飺
��.ȡ����ɫ��Һ5 mL���μ�һ�ΰ�ˮ�г������ɣ��������������ӡ�
��.�ò�˿պȡ��Һ���ڻ��������գ�����ɫ�ܲ����۲죬����ɫ���档
��.��ȡ��Һ����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ��
��.��������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
���ƶϣ�
(1)�ɢ��жϣ���Һ��һ�������е���������____________��
(2)���м�������������ɫ��������ӷ���ʽ��_____________________________��
(3)��ͬѧ����ȷ��ԭ��Һ��������������________����������________�����ݴ��Ʋ�ԭ��ҺӦ�ó�_______________________________________________�ԣ�ԭ����_________________________________(�������ӷ���ʽ˵��)��
(4)��ȡ100 mLԭ��Һ������������NaOH��Һ���˹������漰�����ӷ���ʽΪ__________________________________________________________����ַ�Ӧ����ˣ�ϴ�ӣ����ճ��������أ��õ��Ĺ�������Ϊ________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧϰС����������װ��̽�������백���ķ�Ӧ������A��B�ֱ�Ϊ�����Ͱ����ķ���װ�ã�CΪ��������������백����Ӧ��װ�ã�
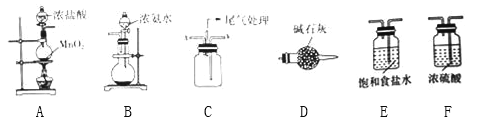
��1����Cl2��������������NH3��������������������ʵ��װ���У��������ӵĺ���˳��Ϊ��A��________________��B��ΪʹNH3��Cl2����ֻ�ϣ�Cl2Ӧ��Cװ�õ�__�ڽ��루�x����y������
��2������װ��A��ʹ�÷�Һ©����Բ����ƿ�еμ�Ũ����IJ�����__________________��
��3��װ��A�з�����Ӧ�����ӷ���ʽΪ_______________________________��
��4��װ��B��Բ����ƿ�ڵ��Լ�Ϊ______________�������ƣ���
��5��װ��D��������____________________________��
��6����Ӧ��ʼ��װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ��һ�������ǿ����е���Ҫ�ɷ�֮һ���÷�Ӧ�Ļ�ѧ����ʽΪ________________________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com