
����Ŀ�����ᣨH2C2O4����һ�ֶ�Ԫ���ᣬ�ڻ�ѧ���������Ҫ����;��ijʵ��Ա��Ҫ�ò��ᾧ�壨H2C2O42H2O������400mL 0.4mol/L��H2C2O4��Һ����ش���������
��1��ͨ�����㣬��ʵ��ԱӦ����������ƽ��______ g H2C2O42H2O������ʵ�顣
��2����д����ʵ���ʵ�鲽��
a:���㣬b������c:_____��d:��ȴ,e:��Һf:ϴ��g:________��h:ҡ�ȣ�i:װҺ��
��3���ڴι������õ��IJ����������˲��������ձ�����ͷ�ι��⣬����_____��
��4���������ƹ����У�����ʵ������������Ƶ���Һ�����ʵ���Ũ���к�Ӱ�죿��ѡ�ƫ�ߡ���ƫ�͡�����Ӱ�족����
I��������ĩʱ���������λ�÷ŷ�����___________��
II����Һ���̺�û�жԲ��������ձ�����ϴ����_________��
III.����ʱ���ӿ̶�����___________��
IV.ҡ��ʱ������ƿҺ����ڿ̶��ߣ��μ�����ˮ���̶�����_________��
���𰸡�25.2 �ܽ� ���� 500mL����ƿ ƫ�� ƫ�� ƫ�� ƫ��
��������
����һ��������Ũ�ȵIJ��裺1.���㣻2.������3.�ܽ⣻4.��ȴ��5.��Һ��6.ϴ�ӣ�7.���ݣ�8.ҡ�ȣ�9.װƿ���õ��������У�������ƽ��ҩ�ס��ձ���Ͳ����������������ƿ����ͷ�ιܣ�����n=cV����������������������������������ݸ���c= ![]() �ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ���n����Һ�����V ����ģ�������ʱ���ؼ�Ҫ�����ƹ���������n��V �����ı仯���Դ˽��
�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ���n����Һ�����V ����ģ�������ʱ���ؼ�Ҫ�����ƹ���������n��V �����ı仯���Դ˽��
��1��ʵ����������400mL0.4mol/L��H2C2O4��Һ����Ҫѡ��500mL����ƿ��ʵ�������Ƶ���500mL 0.4mol/L��H2C2O4��Һ����ҪH2C2O4�����ʵ���Ϊ��0.4mol/L��0.5L=0.2mol����ҪH2C2O42H2O�����ʵ���Ҳ��0.2mol������Ϊ��126g/mol��0.2mol=25.2g��
��2������500mL 0.4mol/L��H2C2O4��Һ�IJ��裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װҺ��
��3������һ�����ʵ���Ũ����Һһ�㲽��Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�ȣ��õ��������У�������ƽ��ҩ�ס��ձ���Ͳ����������������ƿ����ͷ�ιܣ�ȱ�ٵ�����Ϊ��500mL����ƿ��
��4��I.��������̷����壬���̷����룬����֪��m(����)+m(����)=m(����)����֪�������Ƶ�������ʵ��Ҫ�Ƶ�����Ҫ�ᣬ��ʹ������Һ�����ʵ���Ũ��ƫ�ͣ�
II����Һ���̺�û�жԲ��������ձ�����ϴ�ӣ�����������ʧ����ʹ������Һ�����ʵ���Ũ��ƫ�ͣ�
III.����ʱ���ӿ̶��ߣ��������Һ���ƫС����ʹ������Һ�����ʵ���Ũ��ƫ�ߣ�
IV.ҡ��ʱ������ƿҺ����ڿ̶��ߣ��μ�����ˮ���̶��ߣ��������Һ���ƫ��ʹ������Һ�����ʵ���Ũ��ƫ�͡�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�У��������뻹ԭ�����ʵ���֮��Ϊ1��2����(����)
A. 3S��6NaOH===2Na2S��Na2SO3��3H2O
B. 2CH3COOH��Ca(ClO)2===2HClO��Ca(CH3COO)2
C. 4HCl(Ũ)��MnO2![]() MnCl2��Cl2����2H2O
MnCl2��Cl2����2H2O
D. I2��2NaClO3===2NaIO3��Cl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��Ϊ��ѧ��ѧ�ij��������Ҿ�����ͬһ��Ԫ�أ�����֮���ת����ϵ����ͼ��ʾ(��Ӧ���������������Ѿ���ȥ)��
A![]() B
B![]() C
C![]() D��
D��
��1����A��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ������D��ϡ��Һ��ʹʪ�����ɫʯ����ֽ��죬��AΪ____��д��A��Bת���Ļ�ѧ����ʽ��______________��
��2����AΪ���ý���Ԫ�صĵ��ʣ�DΪǿ���ɫ��Ӧ�Ի�ɫ����C��______��A��ˮ������Ӧ�����ӷ���ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��ӦA2��B2 =2AB�������仯��ͼ��ʾ������˵����ȷ����( )
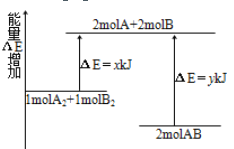
A. �÷�Ӧ�����ȷ�Ӧ
B. 2 mol A��B��������Ҫ����y kJ ������
C. 1 mol A��A����1mol B��B�������ܷų�x kJ������
D. 2 mol AB������������1 mol A2��1 mol B2��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����16gO2����ԭ��������ͬ��NH3��״���������________L��
��2����֪2L Al2(SO4)3��Һ��c(Al3+)=3mol/L������3L__________mol/LNa2SO4��SO42�������ʵ���Ũ����ȡ�
��3��ͬ��ͬѹ�£�ͬ�����NH3��H2S�����������Ϊ_____________��ͬ������NH3��H2S����������Ϊ___________�����к��е����ԭ�Ӹ�����Ϊ________����NH3��H2S�е���ԭ������ȣ����ǵ������Ϊ____________��
��4���ڱ�״���£�8.96L��CH4��CO�Ļ�����壬����������������ܶ���9.5������������CH4�����Ϊ____________��CH4��COԭ�Ӹ�����Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ����������ԭ��Ӧ���ǣ�������
A. SO3+ H2O= H2SO4 B. NH4Cl ![]() NH3
NH3![]() +HCl
+HCl![]()
C. Fe+CuSO4= FeSO4+Cu D. NaOH+HNO3=NaNO3+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ɸ�������ɺ����ʽ��з��ࣺ
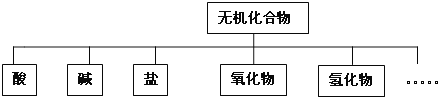
��1��������ʾ�����ʷ���������� ______ ��
��2����CO2 ��Cu ��FeCl3��Һ ��H2SO4 �����������彺�� ��Al2(SO4)3���� ���ƾ� ��BaSO4����
���ڵ���ʵ��� ______ �����ڷǵ���ʵ��� ______ ������ţ���
��3����д�����ĵ��뷽��ʽ______________________________________________
��4�����й�����������˵����ȷ����____________��
a�������ȶ����ܷ⾲�û��������
b�����ܲ��������ЧӦ����������
c���������ᶼ���������
��5����Ҫ��д�����з�Ӧ�����ӷ���ʽ��
��п��ϡ���ᷴӦ_________________________________________________��
������������Һ��ϡ���ᷴӦ ______________________________________��
��MgO�μ�ϡ����_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��д��ȷ����(����)
A. FeCl3��Һ��ʴӡˢ��·ͭ�壺Cu��Fe3��=Cu2����Fe2��
B. �����ܽ⼦���ǣ�2H����CaCO3=Ca2����CO2����H2O
C. ��NaHSO4��Һ�е���Ba(OH)2��Һ����Һ�����ԣ�Ba2����2OH����2H����SO![]() =BaSO4����2H2O
=BaSO4����2H2O
D. ��NaHCO3��Һ�е�����������ʯ��ˮ��HCO![]() ��Ca2����OH��=CaCO3����H2O
��Ca2����OH��=CaCO3����H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2019 ���ǡ����ʻ�ѧԪ�����ڱ��ꡱ�� 1869 ���Ž��з�ѵ�ʱ��֪��Ԫ�ظ�����������ѧ���ʽ������У�ȷԤ���˼ס�������δ֪Ԫ�ص�λ�ã���Ԥ���˶��ߵ����ԭ������������ԭʼ��¼���¡�����˵���д������
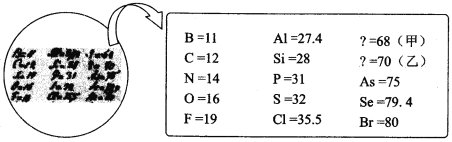
A. ��λ������Ԫ�����ڱ��������ڵڢ�A ��B. ԭ�Ӱ뾶�Ƚϣ��ף��ң� Si
C. �ҵļ���̬�⻯����ȶ���ǿ��CH4D. �Ʋ��ҵĵ��ʿ��������뵼�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com