
����Ŀ��ʵ������һƿ���õİ�ɫ������ط�ĩ��Ϊȷ�����Ƿ���������ɷ֣�Ԫ�ط���������ĩ�м�Ԫ�غ���Ԫ�ص�������Ϊ39��16�����н�����ȷ����(����)
A.����Ԫ�ط�������Ʋ�÷�ĩΪ������
B.����ĩ����ˮ�������Ȼ������а�ɫ�������ɣ�֤��ԭ��ĩ�������
C.����ĩ���������У��������ݣ�֤��ԭ��ĩ���������
D.����ĩ����ˮ�������Ȼ������������ᣬ�а�ɫ�������������� ��֤��ԭ��ĩ������غ�������صĻ����
���𰸡�D
��������
A. ����غ���������м�Ԫ�غ���Ԫ��Ԫ�ص������Ⱦ�Ϊ39��16�������Ʋ�÷�ĩΪ�������A����
B. �����Ȼ������ɲ�����������B����
C. ����ĩ���������У��������ݣ� 2H����![]() ===H2O��SO2����ֻ��֤��ԭ��ĩ��������أ�����֤��ԭ��ĩֻ��������أ���C����
===H2O��SO2����ֻ��֤��ԭ��ĩ��������أ�����֤��ԭ��ĩֻ��������أ���C����
D. ����ĩ����ˮ�������Ȼ��������������а�ɫ������֤��ԭ��ĩ������أ����������ɣ�˵��������Ӧ2HCl��BaSO3=BaCl2��H2O��SO2����֤��ԭ��ĩ�Ժ�������أ���D��ȷ��
��ѡD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����С��������CuO��NH3��Ӧ���о�NH3��ij�����ʲ��ⶨ����ɣ����������ʵ��װ�ã��г�װ��δ����������ʵ�顣��ش��������⣺

��1������a������Ϊ______������b�п�ѡ����Լ�Ϊ______��
��2��ʵ�����У�����װ��A��������ȡ����ɫ������______������ĸ����
A��Cl2 B��O2 C��CO2 D��NO2
��3��ʵ���й۲쵽װ��C�к�ɫCuO��ĩ��Ϊ��ɫ���壬����������ɫ��ζ�����壬��������֤��NH3����______�ԣ�д����Ӧ�Ļ�ѧ����ʽ______��
��4��Eװ����Ũ���������______��
��5����ȡ�������ǰ��Ӧ��װ��F���еIJ�����______��
��6��ʵ����ϣ�����ø����D����mg��װ��F�����������Ϊn L��������ɱ�״�����������е������ԭ�Ӹ�����Ϊ______���ú�m��n��ĸ�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�У����������뻹ԭ����Ϊͬһ�����ʵ���
A.KClO3 + 6HCl = KCl+ 3Cl2��+ 3H2OB.2Na+2H2O=2NaOH+H2��
C.2Na2O2+2H2O=4NaOH+O2��D.I2��2Na2S2O3 = 2NaI �� Na2S4O6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��������Ʒ�к�������������������ijͬѧҪ�ⶨ������Ԫ�ص��������������������ʵ�鷽����

(1)��������������Һʱ�����õ��IJ����������ձ�����Ͳ������������ͷ�ι����⣬��������__________(����������)��
(2)��Ӧ���м�������H2O2��Һ����Ӧ�����ӷ���ʽ��_______________________________��
(3)���������![]() �Ƿ�ϴ�Ӹɾ��IJ�����_______________________________________��
�Ƿ�ϴ�Ӹɾ��IJ�����_______________________________________��
(4)���������ȣ���ȴ�����£�����ƽ������������Ⱥ�����������Ϊb1g���ٴμ��Ȳ���ȴ�����³�������Ϊb2g����b1��b2��0.3����Ӧ���еIJ�����_____________________��
(5)����������Ϊ42.6 g��������������Ⱥ�Ĺ����������Ϊ45.8 g������Ʒ����Ԫ�ص���������Ϊ________________��
(6)��ͬѧ��Ϊ����������ʵ�鲽��̫����������Ϊ��ֻҪ����Ʒ����ˮ��ֽ��裬���ȡ����ɡ����ճ������ɲ����Ʒ����Ԫ�ص���������������Ϊ������������Ƿ���У�__________(����С������С�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ϣ��дָ����Ӧ�ķ���ʽ��
��1�����������Һ��ѡ����ʵ���Һ���ָ��ת�����Լ������ظ�ѡ����Һ��NH3��H2O��FeSO4��Fe2(SO4)3��HCl��NaCl
��д��SO2��H2SO4�Ļ�ѧ����ʽ��____��
��д��SO2��SO32-�����ӷ���ʽ��____��
��2������������(AlH2P3O10��2H2O)����һ��������ɫ�������ϣ���������������Ҫ�ɷ֣�Al2O3��4SiO2��3H2O�������ʣ�FeO��Fe2O3��Na2O�ȣ�Ϊԭ�Ͼ������������Ʊ���
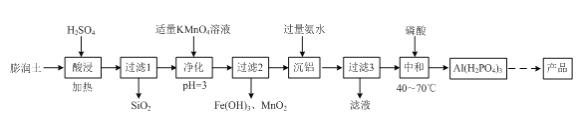
�١������ʱAl2O3��4SiO2��3H2O�����ᷴӦ�Ļ�ѧ����ʽΪ____��
�ڡ�������ʱ������Ӧ�����ӷ���ʽΪ____��
�ۡ�������ʱ������Ӧ�����ӷ���ʽΪ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ����Ũ����ˮ�����ϸ�Ũ�ȵ�Br-��Ϊԭ����ȡ��IJ����������£�
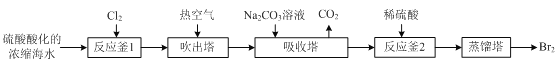
��֪��3Br2+3CO32- =5Br- +BrO3- +3CO2����
ͼ��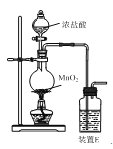 ͼ��
ͼ��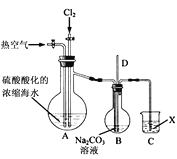
��1����Ӧ�õ�Cl2����ͼ����ʾװ����ȡ����ȡCl2�Ļ�ѧ����ʽΪ____��Ϊ��ȥCl2�лӷ�����HCl��װ��E����ʢ��Һ��____��
��2��ʵ������ͼ����ʾװ��ģ�������еIJ��ֹ��̡�
��ͨ��Cl2ʱ������Ӧ�����ӷ���ʽΪ____��
��ͨ���ȿ�����������____��
���ձ�C����ʢ��Һ��____��
����ƿB�еij�����D����ƽ��ѹǿ�����ã�����ƿ������ѹǿ����ʱ�����Թ۲쵽��������____��
��3������Ӧ��2������������Ӧ�����ӷ���ʽΪ____��
��4���������������Һ������������Br2�����������м�������____��ȡ���е�Br2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ClO2��һ��������ˮ�Ļ���ɫ���壬��������ȫ��Ч��ˮ��������ij��ҺX������ClO2Ũ�ȣ�g��L-1����ʵ��ⶨ�������£�����1. ����Na2S2O3��Һ��ȷ��ȡ1.984 0 g Na2S2O3��5H2O��������в���ȴ������ˮ���100.00 mL��Һ������2. ȷ��ȡ1.00 mL��ҺX������ƿ�У���������ᾲ�ú��ټ����������ἰ�Թ�����KI��Һ(2ClO2��10I-��8H��===5I2��2Cl-��4H2O)���ڰ�������5 min������3. ����2����ƿ�ķ�ӦҺ�м���1 mL������Һ��ָʾ�����������еμӲ���1���Ƶ�Na2S2O3��Һ(������ӦI2��2Na2S2O3===2NaI��Na2S4O6)����ǡ����ȫ��Ӧʱ����Na2S2O3��Һ25.00 mL��
��1������1������Na2S2O3��Һ�����ʵ���Ũ��Ϊ____mol��L-1�����ò����������ձ�����ͷ�ι����____����2������Na2S2O3��Һʱ������ˮ����е�ԭ����____��
��3��������ҺX������ClO2Ũ�ȣ�g��L-1��(д���������)____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ǿƼ���������������Ӧ����㷺�Ľ������ϡ�

�����������Ļ�����������ʵ�飺

(1)����X��������_____��
(2)����A��_____��
(3)A��Cl2��Ϲ��տ��ܷ�����ը������_____(�ѧʽ)��A�ڸ÷�Ӧ����Ϊ_____(������������������ԭ����)��
(4)��ҺB�������ӳ�OH-���_____����ҺD�д��ڵĽ�������Ϊ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������������Ƽ���������أ�����˵��������ǣ� ��
A.��������̫���ܡ����ܺ����ܵ�����Դ���滯ʯ��Դ������ʵ�ֵ�̼����
B.����Ĥ���Ͻ�������ˮ�е���ˮ���룬�������ǻ�ѧ�仯
C.����Cu![]() CuO
CuO![]() Cu(NO3)2�ķ�Ӧ��ȡCu(NO3)2���ϲ��ײ�����Ⱦ����ɫ��ѧ����
Cu(NO3)2�ķ�Ӧ��ȡCu(NO3)2���ϲ��ײ�����Ⱦ����ɫ��ѧ����
D.�����ϴ���������ȡ�����ض�����ű������Ч
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com